Label: NEUROLITE- bicisate dihydrochloride kit
- NDC Code(s): 11994-002-01, 11994-004-01, 11994-006-02, 11994-006-05
- Packager: Lantheus Medical Imaging, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTIONThis kit formulation consists of two nonradioactive vials: Vial A contains bicisate dihydrochloride (N, N'-1,2-ethylenediylbis-L-cysteine diethyl ester dihydrochloride) and a reducing agent as a ...
-
PHYSICAL CHARACTERISTICSTechnetium Tc99m decays by isomeric transition with a physical half-life of 6.02 hrs1. Photons useful for the detection and imaging studies are listed in Table 1. Table 1. Principle ...
-
CLINICAL PHARMACOLOGYGeneral - Neurolite®, Kit for the Preparation of Technetium Tc99m Bicisate for Injection forms a stable, lipophilic complex which can cross the blood brain barrier. Technetium Tc99m Bicisate ...
-
INDICATIONSNeurolite single photon emission computerized tomography (SPECT) is indicated as an adjunct to conventional CT or MRI imaging in the localization of stroke in patients in whom stroke has already ...
-
CONTRAINDICATIONSNone known.
-
WARNINGSNone known.
-
PRECAUTIONSGeneral - USE WITH CAUTION IN PATIENTS WITH RENAL OR HEPATIC IMPAIRMENT. TECHNETIUM Tc99m BICISATE IS ELIMINATED PRIMARILY BY RENAL EXCRETION. WHETHER TECHNETIUM Tc99m BICISATE IS DIALYZABLE IS ...
-
ADVERSE REACTIONSIn clinical trials, Neurolite has been administered to 1063 subjects (255 normals, 808 patients). Of these, 566 (53%) were men and 494 (47%) were women. The mean age was 58 years (range 17 to 92 ...
-
DOSAGE AND ADMINISTRATIONBefore administration, a patient should be well hydrated. After administration, the patient should be encouraged to drink fluids liberally and to void frequently. The recommended dose range for ...
-
RADIATION DOSIMETRYThe radiation doses to organs and tissues of an average patient (70 kg) for Technetium Tc99m Bicisate injected intravenously for 370 MBq (10 mCi) are shown in Table 4 and for 1110 MBq (30 mCi) are ...
-
INSTRUCTIONS FOR PREPARATION OF TECHNETIUM Tc99m BICISATEPreparation of the Technetium Tc99m Bicisate from the NEUROLITE®, Kit for the Preparation of Technetium Tc99m Bicisate Injection, is done by the following aseptic procedure: Prior to adding the ...
-
DETERMINATION OF RADIOCHEMICAL PURITY The preparation and quality control of the agent should follow the procedure shown below. Materials for TLC Procedure - Bakerflex silica gel IB-F, 2.5 x 7.5cm, Baker ...
-
HOW SUPPLIEDLantheus Medical Imaging, Inc. Neurolite® Kit for the Preparation of Technetium Tc99m Bicisate for Injection, is supplied in kits of two (2) vials of A and two (2) vials of B (NDC ...
-
SPL UNCLASSIFIED SECTIONDistributed By - Lantheus Medical Imaging - 331 Treble Cove Road - N. Billerica, Massachusetts 01862 - USA - For Ordering Tel: Toll Free 800-299-3431 - All Other Business: 800-362-2668 - (For Massachusetts ...
-
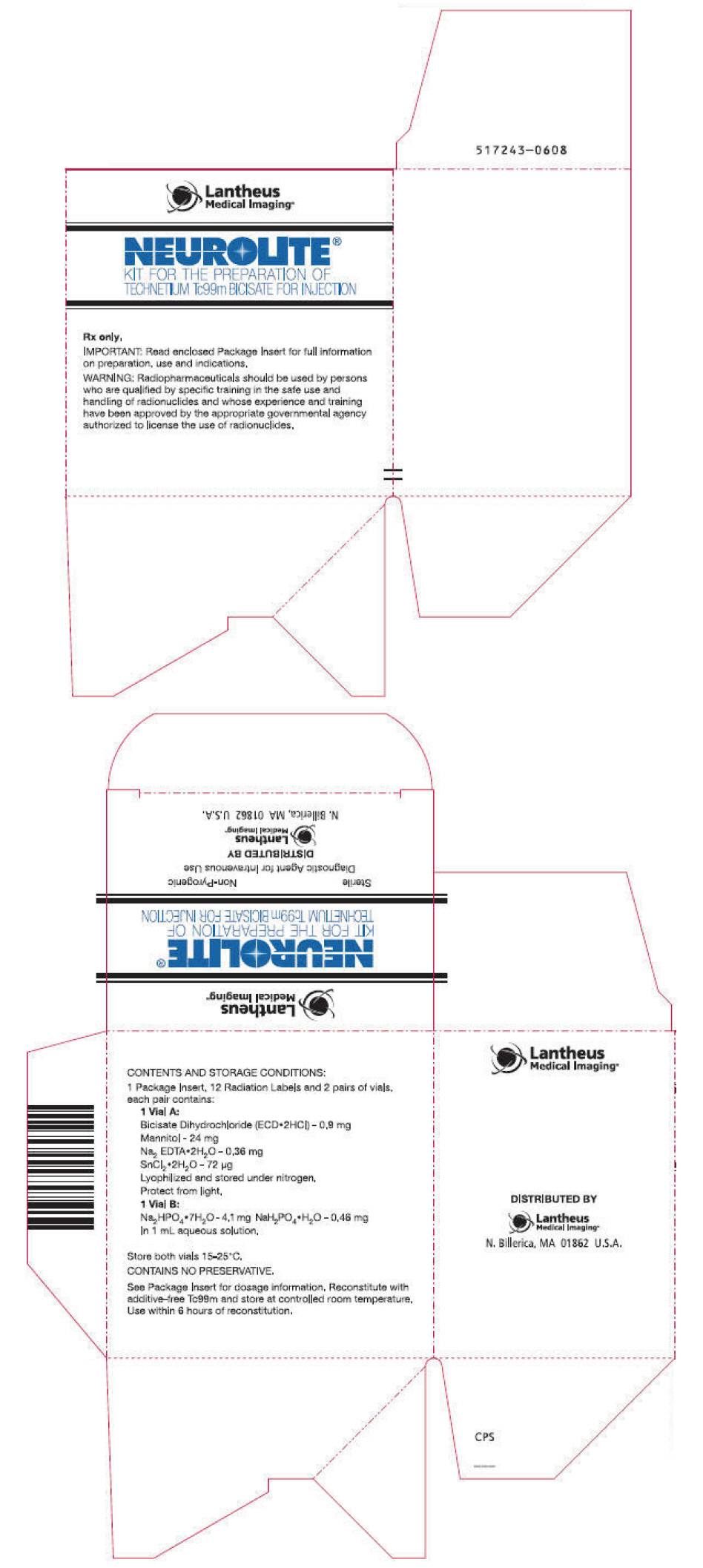
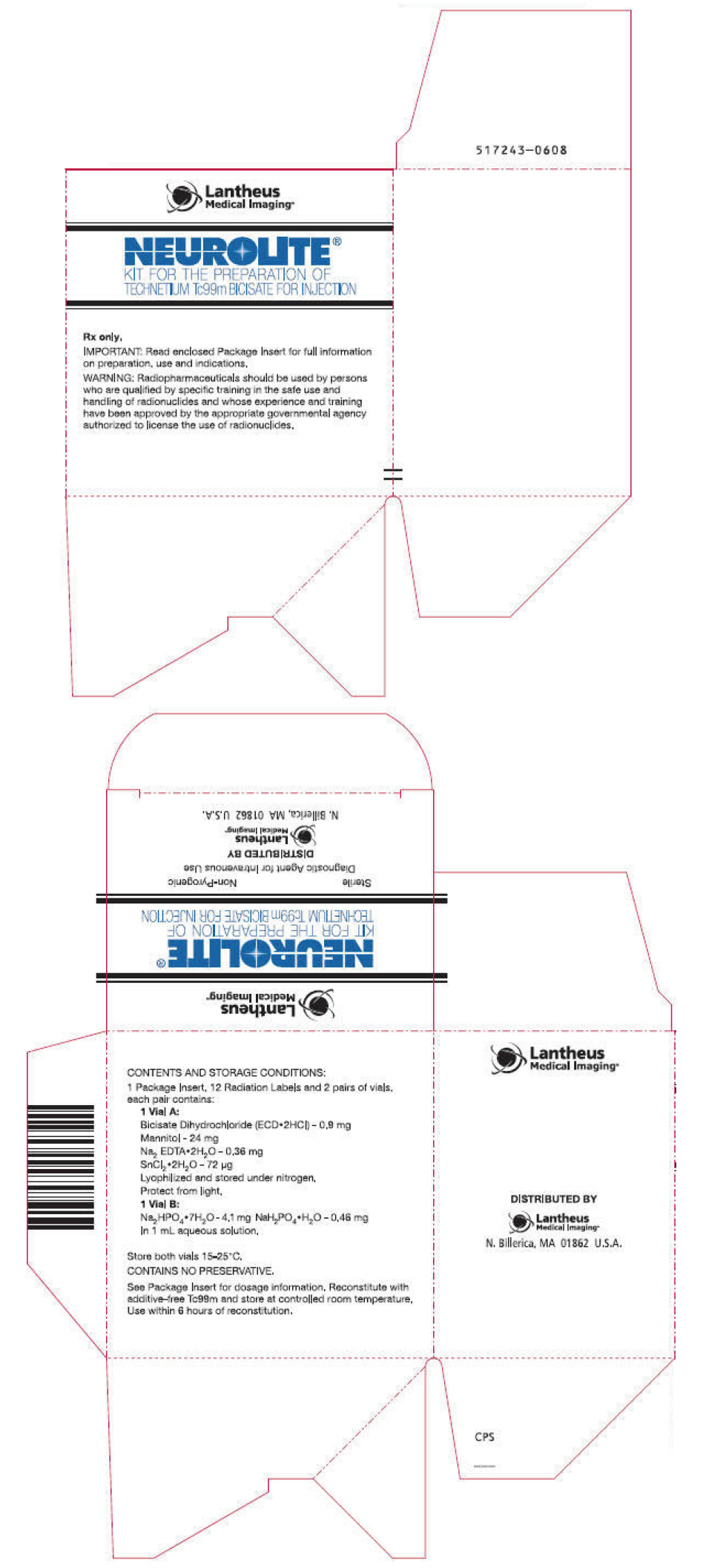
PRINCIPAL DISPLAY PANEL - Kit PackageLantheus - Medical Imaging® NEUROLITE® KIT FOR THE PREPARATION OF - TECHNETIUM Tc99m BICISATE FOR INJECTION - Rx only, IMPORTANT: Read enclosed Package Insert for full information - on preparation, use ...
-
INGREDIENTS AND APPEARANCEProduct Information