Label: BEYAZ- drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit
-
NDC Code(s):
50419-407-00,
50419-407-01,
50419-407-03,
50419-407-70, view more50419-407-71, 50419-407-75
- Packager: Bayer HealthCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BEYAZ safely and effectively. See full prescribing information for BEYAZ. BEYAZ (drospirenone/ethinyl estradiol/levomefolate calcium ...These highlights do not include all the information needed to use BEYAZ safely and effectively. See full prescribing information for BEYAZ.
BEYAZ (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets), for oral use
Initial U.S. Approval: 2010WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
See full prescribing information for complete boxed warning.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Beyaz is a combination of drospirenone, a progestin and ethinyl estradiol, an estrogen containing a folate, indicated for use by females of reproductive potential to:
- •
- Prevent pregnancy. (1.1)
- •
- Treat symptoms of premenstrual dysphoric disorder (PMDD) for females of reproductive potential who choose to use an oral contraceptive for contraception. (1.2)
- •
- Treat moderate acne for females of reproductive potential at least 14 years old only if the patient desires an oral contraceptive for birth control. (1.3)
- •
- Raise folate levels in females of reproductive potential who choose to use an oral contraceptive for contraception. (1.4)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Beyaz consists of 28 film-coated, biconvex tablets in the following order (3):
- •
- 24 pink tablets, each containing 3 mg drospirenone (DRSP), 0.02 mg ethinyl estradiol (EE) as betadex clathrate and 0.451 mg levomefolate calcium
- •
- 4 light orange tablets, each containing 0.451 mg levomefolate calcium
CONTRAINDICATIONS
- •
- Renal impairment (4)
- •
- Adrenal insufficiency (4)
- •
- A high risk of arterial or venous thrombotic diseases (4)
- •
- Undiagnosed abnormal uterine bleeding (4)
- •
- Breast cancer (4)
- •
- Liver tumors or liver disease (4)
- •
- Co-administration with Hepatitis C drug combinations containing ombitasvir, paritaprevir/ritonavir, with or without dasabuvir (4)
WARNINGS AND PRECAUTIONS
- •
- Vascular risks: Stop Beyaz if a thrombotic event occurs. Stop at least 4 weeks before and through 2 weeks after major surgery. Start no earlier than 4 weeks after delivery, in women who are not breastfeeding. (5.1) COCs containing DRSP may be associated with a higher risk of venous thromboembolism (VTE) than COCs containing levonorgestrel or some other progestins. Before initiating Beyaz in a new COC user or a woman who is switching from a contraceptive that does not contain DRSP, consider the risks and benefits of a DRSP-containing COC in light of her risk of a VTE. (5.1)
- •
- Hyperkalemia: DRSP has anti-mineralocorticoid activity. Do not use in patients predisposed to hyperkalemia. Check serum potassium concentration during the first treatment cycle in women on long-term treatment with medications that may increase serum potassium concentration. (5.2, 7.1, 7.2)
- •
- Liver disease: Discontinue Beyaz if jaundice occurs. (5.4)
- •
- High blood pressure: Do not prescribe Beyaz for women with uncontrolled hypertension or hypertension with vascular disease. (5.6)
- •
- Carbohydrate and lipid metabolic effects: Monitor prediabetic and diabetic women taking Beyaz. Consider an alternate contraceptive method for women with uncontrolled dyslipidemia. (5.8)
- •
- Headache: Evaluate significant change in headaches and discontinue Beyaz if indicated. (5.9)
- •
- Uterine bleeding: Evaluate irregular bleeding or amenorrhea. (5.10)
ADVERSE REACTIONS
- •
- The most frequent adverse reactions (≥ 2%) in contraception, acne and folate clinical trials are headache/migraine (5.9%), menstrual irregularities (4.1%), nausea/vomiting (3.5%) and breast pain/tenderness (3.2%). (6.1)
- •
- The most frequent adverse reactions (≥ 2%) in PMDD clinical trials are menstrual irregularities (24.9%), nausea (15.8%), headache (13.0%), breast tenderness (10.5%), fatigue (4.2%), irritability (2.8%), decreased libido (2.8%), increased weight (2.5%), and affect lability (2.1%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- •
- Drugs or herbal products that induce certain enzymes (for example, CYP3A4) may decrease the effectiveness of COCs or increase breakthrough bleeding. Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with COCs. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
1 INDICATIONS AND USAGE
1.1 Oral Contraceptive
1.2 Premenstrual Dysphoric Disorder (PMDD)
1.3 Acne
1.4 Folate Supplementation
2 DOSAGE AND ADMINISTRATION
2.1 How to Take Beyaz
2.2 How to Start Beyaz
2.3 Missed Doses
2.4 Advice in Case of Gastrointestinal Disturbances
2.5 Folate Supplementation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic Disorders and Other Vascular Problems
5.2 Hyperkalemia
5.3 Malignant Neoplasms
5.4 Liver Disease
5.5 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
5.6 High Blood Pressure
5.7 Gallbladder Disease
5.8 Carbohydrate and Lipid Metabolic Effects
5.9 Headache
5.10 Bleeding Irregularities
5.11 Depression
5.12 Interference with Laboratory Tests
5.13 Monitoring
5.14 Other Conditions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Combined Oral Contraceptives
7.2 Effects of Combined Oral Contraceptives on Other Drugs
7.3 Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation
7.4 Effects of Folates on Other Drugs
7.5 Effects of Other Drugs on Folates
7.6 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
8.8 Race
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Oral Contraceptive Clinical Trial
14.2 Premenstrual Dysphoric Disorder Clinical Trials
14.3 Acne Clinical Trials
14.4 Folate Supplementation Clinical Trials
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptives (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs should not be used by women who are over 35 years of age and smoke [see Contraindications (4)].
Close -
1 INDICATIONS AND USAGE1.1 Oral Contraceptive - Beyaz® is indicated for use by females of reproductive potential to prevent pregnancy. 1.2 Premenstrual Dysphoric Disorder (PMDD) Beyaz is also indicated for the ...
1.1 Oral Contraceptive
Beyaz® is indicated for use by females of reproductive potential to prevent pregnancy.
1.2 Premenstrual Dysphoric Disorder (PMDD)
Beyaz is also indicated for the treatment of symptoms of premenstrual dysphoric disorder (PMDD) in females of reproductive potential who choose to use an oral contraceptive as their method of contraception. The effectiveness of Beyaz for PMDD when used for more than three menstrual cycles has not been evaluated.
The essential features of PMDD according to the Diagnostic and Statistical Manual-4th edition (DSM-IV) include markedly depressed mood, anxiety or tension, affective lability, and persistent anger or irritability. Other features include decreased interest in usual activities, difficulty concentrating, lack of energy, change in appetite or sleep, and feeling out of control. Physical symptoms associated with PMDD include breast tenderness, headache, joint and muscle pain, bloating and weight gain. In this disorder, these symptoms occur regularly during the luteal phase and remit within a few days following onset of menses; the disturbance markedly interferes with work or school, or with usual social activities and relationships with others. Diagnosis is made by healthcare providers according to DSM-IV criteria, with symptomatology assessed prospectively over at least two menstrual cycles. In making the diagnosis, care should be taken to rule out other cyclical mood disorders.
Beyaz has not been evaluated for the treatment of premenstrual syndrome (PMS).
1.3 Acne
Beyaz is indicated for the treatment of moderate acne vulgaris in females of reproductive potential at least 14 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche. Beyaz should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control.
Close1.4 Folate Supplementation
Beyaz is indicated in females of reproductive potential who choose to use an oral contraceptive as their method of contraception, to raise folate levels for the purpose of reducing the risk of a neural tube defect in a pregnancy conceived while taking the product or shortly after discontinuing the product.
-
2 DOSAGE AND ADMINISTRATION2.1 How to Take Beyaz - Take one tablet by mouth at the same time every day. The failure rate may increase when pills are missed or taken incorrectly. To achieve maximum contraceptive and PMDD ...
2.1 How to Take Beyaz
Take one tablet by mouth at the same time every day. The failure rate may increase when pills are missed or taken incorrectly.
To achieve maximum contraceptive and PMDD effectiveness, Beyaz must be taken as directed, in the order directed on the blister pack. Single missed pills should be taken as soon as remembered.
2.2 How to Start Beyaz
Instruct the patient to begin taking Beyaz either on the first day of her menstrual period (Day 1 Start) or on the first Sunday after the onset of her menstrual period (Sunday Start).
Day 1 Start
During the first cycle of Beyaz use, instruct the patient to take one pink Beyaz daily, beginning on Day 1 of her menstrual cycle. (The first day of menstruation is Day 1.) She should take one pink Beyaz daily for 24 consecutive days, followed by one light orange tablet daily on Days 25 through 28. Beyaz should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Beyaz can be taken without regard to meals. If Beyaz is first taken later than the first day of the menstrual cycle, Beyaz should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
Sunday Start
During the first cycle of Beyaz use, instruct the patient to take one pink Beyaz daily, beginning on the first Sunday after the onset of her menstrual period. She should take one pink Beyaz daily for 24 consecutive days, followed by one light orange tablet daily on Days 25 through 28. Beyaz should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Beyaz can be taken without regard to meals. Beyaz should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
The patient should begin her next and all subsequent 28-day regimens of Beyaz on the same day of the week that she began her first regimen, following the same schedule. She should begin taking her pink tablets on the next day after ingestion of the last light orange folate tablet, regardless of whether or not a menstrual period has occurred or is still in progress. Anytime a subsequent cycle of Beyaz is started later than the day following administration of the last light orange tablet, the patient should use another method of contraception until she has taken a pink Beyaz daily for seven consecutive days.
When switching from a different birth control pill
When switching from another birth control pill, Beyaz should be started on the same day that a new pack of the previous oral contraceptive would have been started.
When switching from a method other than a birth control pill
When switching from a transdermal patch or vaginal ring, Beyaz should be started when the next application would have been due. When switching from an injection, Beyaz should be started when the next dose would have been due. When switching from an intrauterine contraceptive or an implant, Beyaz should be started on the day of removal.
Withdrawal bleeding usually occurs within 3 days following the last pink tablet. If spotting or breakthrough bleeding occurs while taking Beyaz, instruct the patient to continue taking Beyaz by the regimen described above. Counsel her that this type of bleeding is usually transient and without significance; however, advise her that if the bleeding is persistent or prolonged, she should consult her healthcare provider.
Although the occurrence of pregnancy is low if Beyaz is taken according to directions, if withdrawal bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy. Discontinue Beyaz if pregnancy is confirmed.
The risk of pregnancy increases with each active pink tablet missed. If breakthrough bleeding occurs following missed tablets, it will usually be transient and of no consequence. If the patient misses one or more light orange tablets, she should still be protected against pregnancy provided she begins taking a new cycle of pink tablets on the proper day.
For postpartum women who do not breastfeed or after a second trimester abortion, start Beyaz no earlier than 4 weeks postpartum due to the increased risk of thromboembolism. If the patient starts on Beyaz postpartum and has not yet had a period, evaluate for possible pregnancy, and instruct her to use an additional method of contraception until she has taken Beyaz for 7 consecutive days.
2.3 Missed Doses
Table 1: Instructions for Beyaz Missed Doses If one pink active tablet is missed
Take it as soon as possible. Take the next tablet at the regular time. This means two tablets may be taken in one day. A back-up birth control method is not required if the patient has sex.
If two pink active tablets in a row are missed in Week 1 or Week 2
Take two tablets as soon as possible and two tablets the next day. Then take one tablet a day until the pack is finished. Additional nonhormonal contraception (such as condoms and spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets.
If two pink active tablets in a row are missed in Week 3 or Week 4
Day 1 Start: Throw out the rest of the pack and start a new pack that same day.
Sunday Start: Keep taking one tablet every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack that same day.
Additional nonhormonal contraception (such as condoms and spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. The patient may not have their period this month but this is expected. However, if they miss their period two months in a row, they should call their healthcare provider because they might be pregnant.
If three or more pink active tablets in a row are missed during any week
Day 1 Start: Throw out the rest of the pack and start a new pack that same day.
Sunday Start: Keep taking one tablet every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack that same day.
Additional nonhormonal contraception (such as condoms and spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. The patient may not have their period this month but this is expected. However, if they miss their period two months in a row, they should call their healthcare provider because they might be pregnant.
If any of the four light orange inactive tablets are missed in Week 4:
Throw away the tablets that were missed.
Keep taking one tablet each day until the pack is empty. They do not need a back-up method.
Finally, if they are still not sure what to do about the tablets they have missed:
Use nonhormonal contraception (such as condoms and spermicides) anytime they have sex. Contact their healthcare provider and continue taking one active pink tablet each day until otherwise directed.
2.4 Advice in Case of Gastrointestinal Disturbances
In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting occurs within 3-4 hours after tablet-taking, this can be regarded as a missed tablet.
Close2.5 Folate Supplementation
The U.S. Preventive Services Task Force recommends that women of childbearing age consume supplemental folic acid in a dose of at least 0.4 mg (400 mcg) daily.1 Consider other folate supplementation that a woman may be taking before prescribing Beyaz. Ensure that folate supplementation is maintained if a woman discontinues Beyaz due to pregnancy.
-
3 DOSAGE FORMS AND STRENGTHSBeyaz (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) is available in blister packs. Each blister pack (28 film-coated tablets) contains in the ...
Beyaz (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) is available in blister packs.
Each blister pack (28 film-coated tablets) contains in the following order:
- •
- 24 pink tablets each containing 3 mg drospirenone (DRSP), 0.02 mg ethinyl estradiol (EE) as betadex clathrate and 0.451 mg levomefolate calcium
- •
- 4 light orange tablets each containing 0.451 mg levomefolate calcium
-
4 CONTRAINDICATIONSBeyaz is contraindicated in females who are known to have or develop the following conditions: • Renal impairment - • Adrenal insufficiency - • A high risk of arterial or venous thrombotic diseases ...
Beyaz is contraindicated in females who are known to have or develop the following conditions:
- •
- Renal impairment
- •
- Adrenal insufficiency
- •
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- •
- Smoke, if over age 35 [see Boxed Warning and Warnings and Precautions (5.1)]
- •
- Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions (5.1)]
- •
- Have cerebrovascular disease [see Warnings and Precautions (5.1)]
- •
- Have coronary artery disease [see Warnings and Precautions (5.1)]
- •
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)]
- •
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)]
- •
- Have uncontrolled hypertension [see Warnings and Precautions (5.6)]
- •
- Have diabetes mellitus with vascular disease [see Warnings and Precautions (5.8)]
- •
- Have headaches with focal neurological symptoms or have migraine headaches with or without aura if over age 35 [see Warnings and Precautions (5.9)]
- •
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.10)]
- •
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive [see Warnings and Precautions (5.3)]
- •
- Liver tumors, benign or malignant, or liver disease [see Warnings and Precautions (5.4) and Use in Specific Populations (8.7
- •
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir due to the potential for ALT elevations [see Warnings and Precautions (5.5) and Drug Interactions (7.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Thromboembolic Disorders and Other Vascular Problems - Stop Beyaz if an arterial or venous thrombotic (VTE) event occurs. Based on presently available information on DRSP-containing COCs ...
5.1 Thromboembolic Disorders and Other Vascular Problems
Stop Beyaz if an arterial or venous thrombotic (VTE) event occurs.
Based on presently available information on DRSP-containing COCs with 0.03 mg ethinyl estradiol (that is, Yasmin), DRSP-containing COCs may be associated with a higher risk of venous thromboembolism (VTE) than COCs containing the progestin levonorgestrel or some other progestins. Epidemiologic studies that compared the risk of VTE reported that the risk ranged from no increase to a three-fold increase. Before initiating use of Beyaz in a new COC user or a woman who is switching from a contraceptive that does not contain DRSP, consider the risks and benefits of a DRSP-containing COC in light of her risk of a VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs [see Contraindications (4)].
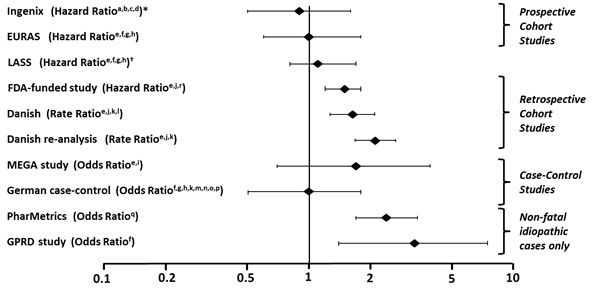
A number of studies have compared the risk of VTE for users of Yasmin (which contains 0.03 mg of EE and 3 mg of DRSP) to the risk for users of other COCs, including COCs containing levonorgestrel. Those that were required or sponsored by regulatory agencies are summarized in Table 2.
Table 2: Estimates (Hazard Ratios) of Venous Thromboembolism Risk in Current Users of Yasmin Compared to Users of Oral Contraceptives that Contain Other Progestins - *
- "New users" - no use of combination hormonal contraception for at least the prior 6 months
- †
- Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, levonorgestrel, desogestrel, norgestrel, medroxyprogesterone, or ethynodiol diacetate
- ‡
- Includes low-dose COCs containing the following progestins: levonorgestrel, desogestrel, dienogest, chlormadinone acetate, gestodene, cyproterone acetate, norgestimate, or norethindrone
- §
- Includes low-dose COCs containing the following progestins: norgestimate, norethindrone, or levonorgestrel
Epidemiologic Study
(Author, Year of Publication)
Population StudiedComparator Product
(all are low-dose COCs; with ≤ 0.04 mg of EE)Hazard Ratio (HR)
(95% CI)i3 Ingenix
(Seeger 2007) Initiators, including new users*All COCs available in the US
during the conduct of the study†
HR: 0.9
(0.5-1.6)
EURAS
(Dinger 2007)
Initiators, including new users*All COCs available in Europe
during the conduct of the study‡HR: 0.9
(0.6-1.4)Levonorgestrel/EE
HR: 1.0
(0.6-1.8)FDA-funded study” (2011)
New users*
Other COCs available during the course of the study§
HR: 1.8
(1.3-2.4)Levonorgestrel/0.03 mg EE
HR: 1.6
(1.1-2.2)All users
(i.e., initiation and continuing use of study combination hormonal contraception)Other COCs available during the course of the study§
HR: 1.7
(1.4-2.1)Levonorgestrel/0.03 mg EE
HR: 1.5
(1.2-1.8)In addition to these “regulatory studies,” other studies of various designs have been conducted. Overall, there are two prospective cohort studies (see Table 2): the US post-approval safety study Ingenix [Seeger 2007], the European post-approval safety study EURAS (European Active Surveillance Study) [Dinger 2007]. An extension of the EURAS study, the Long-Term Active Surveillance Study (LASS), did not enroll additional subjects, but continued to assess VTE risk. There are three retrospective cohort studies: one study in the US funded by the FDA (see Table 2), and two from Denmark [Lidegaard 2009, Lidegaard 2011]. There are two case-control studies: the Dutch MEGA study analysis [van Hylckama Vlieg 2009] and the German case-control study [Dinger 2010]. There are two nested case-control studies that evaluated the risk of non-fatal idiopathic VTE: the PharMetrics study [Jick 2011] and the GPRD study [Parkin 2011]. The results of all of these studies are presented in Figure 1.
Risk ratios displayed on logarithmic scale; risk ratio < 1 indicates a lower risk of VTE for DRSP, > 1 indicates an increased risk of VTE for DRSP.
*Comparator “Other COCs”, including LNG- containing COCs
† LASS is an extension of the EURAS study
#Some adjustment factors are indicated by superscript letters: a) Current heavy smoking, b) hypertension, c) obesity, d) family history, e) age, f) BMI, g) duration of use, h) VTE history, i) period of inclusion, j) calendar year, k) education, l) length of use, m) parity, n) chronic disease, o) concomitant medication, p) smoking, q) duration of exposure, r) site
(References: Ingenix [Seeger 2007]2, EURAS (European Active Surveillance Study) [Dinger 2007]3, LASS (Long-Term Active Surveillance Study) [Dinger, unpublished document on file], FDA-funded study [Sidney 2011]4, Danish [Lidegaard 2009]5, Danish re-analysis [ Lidegaard 2011]6, MEGA study [van Hylckama Vlieg 2009]7, German Case-Control study [Dinger 2010]8, PharMetrics [Jick 2011]9, GPRD study [Parkin 2011]10)
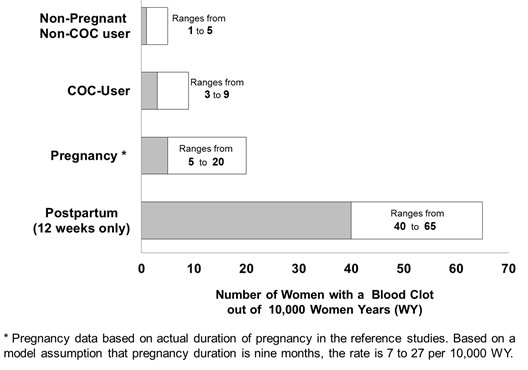
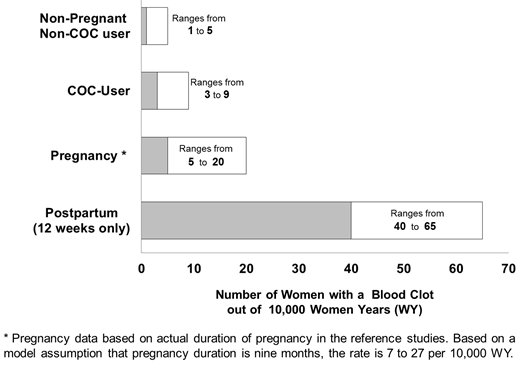
Although the absolute VTE rates are increased for users of hormonal contraceptives compared to non-users, the rates during pregnancy are even greater, especially during the post-partum period (see Figure 2). The risk of VTE in women using COCs has been estimated to be 3 to 9 per 10,000 woman-years. The risk of VTE is highest during the first year of use. Data from a large, prospective cohort safety study of various COCs suggest that this increased risk, as compared to that in non-COC users, is greatest during the first 6 months of COC use. Data from this safety study indicate that the greatest risk of VTE is present after initially starting a COC or restarting (following a 4 week or greater pill-free interval) the same or a different COC.
The risk of thromboembolic disease due to oral contraceptives gradually disappears after COC use is discontinued.
Figure 2 shows the risk of developing a VTE for women who are not pregnant and do not use oral contraceptives, for women who use oral contraceptives, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these women will develop a VTE.
If feasible, stop Beyaz at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
Start Beyaz no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
Use of COCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events.
COCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke. COCs also increase the risk for stroke in women with other underlying risk factors.
Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
Stop Beyaz if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately. [See Adverse Reactions (6).]
5.2 Hyperkalemia
Beyaz contains 3 mg of the progestin DRSP, which has anti-mineralocorticoid activity, including the potential for hyperkalemia in high-risk patients, comparable to a 25 mg dose of spironolactone. Beyaz is contraindicated in patients with conditions that predispose to hyperkalemia (that is, renal impairment, hepatic impairment, and adrenal insufficiency). Women receiving daily, long-term treatment for chronic conditions or diseases with medications that may increase serum potassium concentration should have their serum potassium concentration checked during the first treatment cycle. Medications that may increase serum potassium concentration include ACE inhibitors, angiotensin-II receptor antagonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDs. Consider monitoring serum potassium concentration in high-risk patients who take a strong CYP3A4 inhibitor long-term and concomitantly. Strong CYP3A4 inhibitors include azole antifungals (e.g. ketoconazole, itraconazole, voriconazole), HIV/HCV protease inhibitors (e.g., indinavir, boceprevir), and clarithromycin [see Clinical Pharmacology (12.3)].
5.3 Malignant Neoplasms
Breast Cancer
Beyaz is contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications (4)].
Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see Adverse Reactions (6.2)].
5.4 Liver Disease
Discontinue Beyaz if jaundice develops. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded.
Hepatic adenomas are associated with COC use. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) COC users. However, the attributable risk of liver cancers in COC users is less than one case per million users.
Oral contraceptive-related cholestasis may occur in women with a history of pregnancy-related cholestasis. Women with a history of COC-related cholestasis may have the condition recur with subsequent COC use.
5.5 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as COCs. Discontinue Beyaz prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4)]. Beyaz can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.6 High Blood Pressure
For women with well-controlled hypertension, monitor blood pressure and stop Beyaz if blood pressure rises significantly. Women with uncontrolled hypertension or hypertension with vascular disease should not use COCs.
An increase in blood pressure has been reported in women taking COCs, and this increase is more likely in older women and with extended duration of use. The incidence of hypertension increases with increasing concentration of progestin.
5.7 Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among COC users.
5.8 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who are taking Beyaz. COCs may decrease glucose tolerance in a dose-related fashion.
Consider alternative contraception for women with uncontrolled dyslipidemia. A small proportion of women will have adverse lipid changes while on COCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
5.9 Headache
If a woman taking Beyaz develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue Beyaz if indicated.
An increase in frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation of the COC.
5.10 Bleeding Irregularities
Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on COCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different COC.
Data for Beyaz show the average number of episodes of bleeding per reference period (90 days) was 3.2 in Cycles 4-6. The average number of bleeding and/or spotting days with Beyaz was 15.1 days. The intensity of bleeding for Beyaz based on the ratio of spotting-only days versus total bleeding and/or spotting days was 5.2/15.1 days.
Based on patient diaries from two contraceptive clinical trials of YAZ, 8 to 25% of women experienced unscheduled bleeding per 28-day cycle. A total of 12 subjects out of 1,056 (1.1%) discontinued YAZ due to menstrual disorders including intermenstrual bleeding, menorrhagia, and metrorrhagia.
Women who use Beyaz may experience absence of withdrawal bleeding, even if they are not pregnant. Based on subject diaries from YAZ contraception trials for up to 13 cycles, 6 to 10% of women experienced cycles with no withdrawal bleeding. Some women may encounter post-pill amenorrhea or oligomenorrhea, especially when such a condition was pre-existent.
If withdrawal bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
5.11 Depression
Women with a history of depression should be carefully observed and Beyaz discontinued if depression recurs to a serious degree.
5.12 Interference with Laboratory Tests
The use of COCs may change the results of some laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins. Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentrations of thyroid-binding globulin increase with use of COCs [see Drug Interactions (7.2)].
DRSP causes an increase in plasma renin activity and plasma aldosterone induced by its mild anti-mineralocorticoid activity.
Folates may mask vitamin B12 deficiency.
5.13 Monitoring
A woman who is taking COCs should have a yearly visit with her healthcare provider for a blood pressure check and for other indicated healthcare.
Close5.14 Other Conditions
In women with hereditary angioedema, exogenous estrogens may induce or exacerbate symptoms of angioedema. Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation while taking COCs.
-
6 ADVERSE REACTIONSThe following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling: • Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions ...
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- •
- Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions (5.1)]
- •
- Vascular events [see Warnings and Precautions (5.1)]
- •
- Liver disease [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in practice.
Contraception, Acne and Folate Supplementation Clinical Trials
The data provided reflect the experience with the use of YAZ (3 mg DRSP/0.02 mg EE), in the adequate and well-controlled studies for contraception (N=1,056), for moderate acne vulgaris (N=536) and folate supplementation (N=379).
For contraception, a Phase 3, multicenter, multinational, open-label study was conducted to evaluate safety and efficacy up to one year in 1,027 women aged 17–36 who took at least one dose of YAZ. A second Phase 3 study was a single center, open-label, active-controlled study to evaluate the effect of 7 28-day cycles of YAZ on carbohydrate metabolism, lipids and hemostasis in 29 women aged 18–35. For acne, two multicenter, double-blind, randomized, placebo-controlled studies, in 536 women aged 14–45 with moderate acne vulgaris who took at least one dose of YAZ, evaluated the safety and efficacy during up to 6 cycles. For folate supplementation, the primary efficacy study using Beyaz was a multicenter, double-blind, randomized, active-controlled US trial in 379 healthy women aged 18–40 who were treated with Beyaz or YAZ for up to 24 weeks.
The adverse reactions seen across the 3 indications overlapped, and are reported using the frequencies from the pooled dataset. The most common adverse reactions (≥ 2% of users) were: headache/migraine (5.9%), menstrual irregularities (including vaginal hemorrhage [primarily spotting], metrorrhagia and menorrhagia) (4.1%), nausea/vomiting (3.5%), and breast pain/tenderness (3.2%).
PMDD Clinical Trials
Safety data from trials for the indication of PMDD are reported separately due to differences in study design and setting in the OC, Acne and Folate Supplementation studies as compared to the PMDD clinical program.
Two (one parallel and one crossover designed) multicenter, double-blind, randomized, placebo-controlled trials for the secondary indication of treating the symptoms of PMDD evaluated safety and efficacy of YAZ during up to 3 cycles among 285 women aged 18–42, diagnosed with PMDD and who took at least one dose of YAZ.
Common adverse reactions (≥ 2% of users) were: menstrual irregularities (including vaginal hemorrhage [primarily spotting] and metrorrhagia) (24.9%), nausea (15.8%), headache (13.0%), breast tenderness (10.5%), fatigue (4.2%), irritability (2.8%), decreased libido (2.8%), increased weight (2.5%), and affect lability (2.1%).
Adverse Reactions (≥1%) Leading to Study Discontinuation:
Contraception Clinical Trials
Of 1,056 women, 6.6% discontinued from the clinical trials due to an adverse reaction; the most frequent adverse reactions leading to discontinuation were headache/migraine (1.6%) and nausea/vomiting (1.0%).
Acne Clinical Trials
Of 536 women, 5.4% discontinued from the clinical trials due to an adverse reaction; the most frequent adverse reaction leading to discontinuation was menstrual irregularities (including menometrorrhagia, menorrhagia, metrorrhagia and vaginal hemorrhage) (2.2%) .
Folate Clinical Trial
Of 285 women, 4.6% who used Beyaz or YAZ discontinued from the clinical trials due to an adverse reaction; no reaction leading to discontinuation occurred in ≥ 1% of women.
PMDD Clinical Trials
Of 285 women, 11.6% discontinued from the clinical trials due to an adverse reaction; the most frequent adverse reactions leading to discontinuation were: nausea/vomiting (4.6%), menstrual irregularity (including vaginal hemorrhage, menorrhagia, menstrual disorder, menstruation irregular and metrorrhagia) (4.2%), fatigue (1.8%), breast tenderness (1.4%), depression (1.4%), headache (1.1%), and irritability (1.1%).
Close6.2 Postmarketing Experience
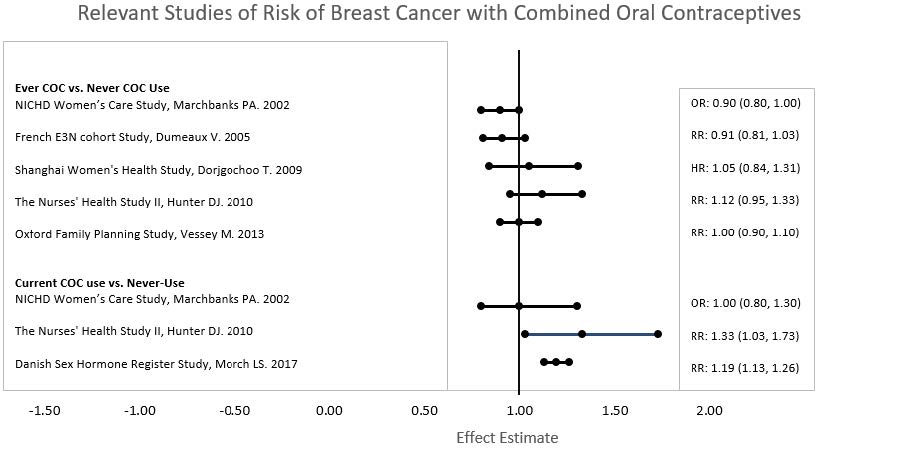
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 3).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 3). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
The following adverse reactions have been identified during post approval use of YAZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions are grouped into System Organ Classes, and ordered by frequency.
Vascular disorders: Venous and arterial thromboembolic events (including pulmonary emboli, deep vein thrombosis, cerebral thrombosis, retinal thrombosis, myocardial infarction and stroke), hypertension (including hypertensive crisis)
Hepatobiliary disorders: Gallbladder disease, liver function disturbances, liver tumors
Immune system disorders: Hypersensitivity (including anaphylactic reaction)
Metabolism and nutrition disorders: Hyperkalemia, hypertriglyceridemia, changes in glucose tolerance or effect on peripheral insulin resistance (including diabetes mellitus)
Skin and subcutaneous tissue disorders: Chloasma, angioedema, erythema nodosum, erythema multiforme
Gastrointestinal disorders: Inflammatory bowel disease
- Musculoskeletal and connective tissue disorders: Systemic lupus erythematosus
-
7 DRUG INTERACTIONSConsult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations. 7.1 Effects of Other ...
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.1 Effects of Other Drugs on Combined Oral Contraceptives
Substances diminishing the efficacy of COCs: Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the effectiveness of COCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampin, topiramate and products containing St. John’s wort. Interactions between oral contraceptives and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative method of contraception or a back-up method when enzyme inducers are used with COCs, and to continue back-up contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Substances increasing the plasma concentrations of COCs: Co-administration of atorvastatin and certain COCs containing EE increase AUC values for EE by approximately 20%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation.
Concomitant administration of moderate or strong CYP3A4 inhibitors such as azole antifungals (e.g., ketoconazole, itraconazole, voriconazole, fluconazole), verapamil, macrolides (e.g., clarithromycin, erythromycin), diltiazem, and grapefruit juice can increase the plasma concentrations of the estrogen or the progestin or both. In a clinical drug-drug interaction study conducted in premenopausal women, once daily co-administration of DRSP 3 mg/EE 0.02 mg containing tablets with strong CYP3A4 inhibitor, ketoconazole 200 mg twice daily for 10 days resulted in a moderate increase of DRSP systemic exposure. The exposure of EE was increased mildly [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
Human immunodeficiency virus (HIV)/Hepatitis C virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma concentrations of estrogen and progestin have been noted in some cases of co-administration with HIV/HCV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
7.2 Effects of Combined Oral Contraceptives on Other Drugs
COCs containing EE may inhibit the metabolism of other compounds. COCs have been shown to significantly decrease plasma concentrations of lamotrigine, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary. Consult the labeling of the concurrently-used drug to obtain further information about interactions with COCs or the potential for enzyme alterations.
COCs Increasing the Plasma Concentrations of CYP450 Enzymes: In clinical studies, administration of a hormonal contraceptive containing EE did not lead to any increase or only to a weak increase in plasma concentrations of CYP3A4 substrates (e.g., midazolam) while plasma concentrations of CYP2C19 substrates (e.g., omeprazole and voriconazole) and CYP1A2 substrates (e.g., theophylline and tizanidine) can have a weak or moderate increase.
Clinical studies did not indicate an inhibitory potential of DRSP towards human CYP enzymes at clinically relevant concentrations [see Clinical Pharmacology (12.3)].
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentration of thyroid-binding globulin increases with use of COCs.
Potential to Increase Serum Potassium Concentration: There is a potential for an increase in serum potassium concentration in women taking Beyaz with other drugs that may increase serum potassium concentration [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
7.3 Concomitant Use with HCV Combination Therapy – Liver Enzyme Elevation
Do not co-administer Beyaz with HCV drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations [see Warnings and Precautions (5.5)].
7.4 Effects of Folates on Other Drugs
Folates may modify the pharmacokinetics or pharmacodynamics of certain antifolate drugs, e.g., antiepileptics (such as phenytoin), methotrexate or pyrimethamine, and may result in a decreased pharmacological effect of the antifolate drug.
7.5 Effects of Other Drugs on Folates
Several drugs have been reported to reduce folate concentrations by inhibition of the dihydrofolate reductase enzyme (e.g., methotrexate and sulfasalazine) or by reducing folate absorption (e.g., cholestyramine), or via unknown mechanisms (e.g., antiepileptics such as carbamazepine, phenytoin, phenobarbital, primidone and valproic acid).
Close7.6 Interference with Laboratory Tests
The use of contraceptive steroids may influence the results of certain laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins. DRSP causes an increase in plasma renin activity and plasma aldosterone induced by its mild anti-mineralocorticoid activity. Folates may mask vitamin B12 deficiency. [See Warnings and Precautions (5.12) and Drug Interactions (7.2).]
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There is no use for contraception in pregnancy; therefore, Beyaz should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not found ...
8.1 Pregnancy
Risk Summary
There is no use for contraception in pregnancy; therefore, Beyaz should be discontinued during pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to CHCs before conception or during early pregnancy. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
Data
Human Data
A retrospective database study of women in Norway, that included 44,734 pregnancies of which 368 were women who inadvertently took drospirenone/ethinyl estradiol during the first trimester of a pregnancy, found there were no adverse effects on pre-term birth, small for gestational age, or birth weight Z-scores.
Post-marketing adverse event data on the use of Beyaz in pregnant women suggest that frequencies of miscarriage and congenital anomalies were not higher than the estimated background risk in the general population.
8.2 Lactation
Risk Summary
DRSP is present in human milk. After a single oral administration of 3 mg DRSP/0.03 mg EE tablets, DRSP concentration in breast milk over the 24-h period ranged from 1.4 to 7.0 ng/mL, with a mean ± standard deviation value of 3.7 ± 1.9 ng/mL.The estimated mean infant dose was 0.003 mg/day, which is about 0.1% of maternal dose (see Data). There is limited information on the effects of Beyaz on the breast-fed infant. CHCs can reduce milk production in breast-feeding females. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breast-feeding. [See also Dosage and Administration (2.2)]. Increase in folate concentration in milk is not expected (see Data).
The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for Beyaz and any potential adverse effects on the breast-fed child from Beyaz or from the underlying maternal condition.
Data
Human Data
An open-label study evaluated the degree of DRSP transfer into milk within 72 hours following a single oral administration of 3 mg DRSP/0.03 mg EE tablets to 6 healthy lactating women who were 1 week to 3 months post-partum. DRSP was present in breast milk with a mean Cmax of 13.5 ng/mL, while the mean Cmax in serum of lactating women was 30.8 ng/mL. The DRSP concentration in breast milk over the 24-hour period following dosing ranged from 1.4 to 7.0 ng/mL, with a mean ± standard deviation value of 3.7 ± 1.9 ng/mL. Based on single dose data, the maximal daily infant dose of DRSP was calculated to be 0.003 mg/day, which represented a mean of 0.1% of the maternal dose.
A study in approximately 60 lactating women demonstrated no significant differences in folate concentrations in milk between women who received 416mcg/day [6S]-5-methyltetrahydrofolate or 400 mcg/day folic acid and women who received placebo over a 16 week period. Studies to date indicate there is no adverse effect of folate on nursing infants.
8.4 Pediatric Use
Safety and efficacy of Beyaz has been established in women of reproductive age. Efficacy is expected to be the same for postpubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
8.5 Geriatric Use
Beyaz has not been studied in postmenopausal women and is not indicated in this population.
8.6 Patients with Renal Impairment
Beyaz is contraindicated in patients with renal impairment [see Contraindications (4) and Warnings and Precautions (5.2)].
In subjects with creatinine clearance (CLcr) of 50–79 mL/min, serum DRSP concentrations were comparable to those in a control group with CLcr ≥ 80 mL/min. In subjects with CLcr of 30–49 mL/min, serum DRSP concentrations were on average 37% higher than those in the control group. In addition, there is a potential to develop hyperkalemia in subjects with renal impairment whose serum potassium is in the upper reference range, and who are concomitantly using potassium-sparing drugs [see Clinical Pharmacology (12.3)].
8.7 Patients with Hepatic Impairment
Beyaz is contraindicated in patients with hepatic disease [see Contraindications (4) and Warnings and Precautions (5.4)]. The mean exposure to DRSP in women with moderate liver impairment is approximately three times higher than the exposure in women with normal liver function. Beyaz has not been studied in women with severe hepatic impairment.
Close8.8 Race
No clinically significant difference was observed between the pharmacokinetics of DRSP or EE in Japanese versus Caucasian women [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEThere have been no reports of serious ill effects from overdose, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea. DRSP is a spironolactone analogue ...
There have been no reports of serious ill effects from overdose, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea.
DRSP is a spironolactone analogue which has anti-mineralocorticoid properties. Serum concentration of potassium and sodium, and evidence of metabolic acidosis, should be monitored in cases of overdose.
Levomefolate calcium doses of 17 mg/day (37-fold higher than the levomefolate calcium dose of Beyaz) were well tolerated after long-term treatment up to 12 weeks.
Close -
11 DESCRIPTIONBeyaz (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) provides an oral contraceptive regimen consisting of 28 film-coated tablets that contain the ...
Beyaz (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) provides an oral contraceptive regimen consisting of 28 film-coated tablets that contain the active ingredients specified for each tablet below:
- •
- 24 pink tablets each containing 3 mg DRSP, 0.02 mg EE as betadex clathrate, and 0.451 mg levomefolate calcium
- •
- 4 light orange tablets each containing 0.451 mg levomefolate calcium
The inactive ingredients in the pink tablets are lactose monohydrate NF, microcrystalline cellulose NF, croscarmellose sodium NF, hydroxypropyl cellulose NF, magnesium stearate NF, hypromellose USP, titanium dioxide USP, talc USP, polyethylene glycol NF, ferric oxide pigment, red NF. The light orange film-coated tablets contain 0.451 mg of levomefolate calcium. The inactive ingredients in the light orange tablets are lactose monohydrate NF, microcrystalline cellulose NF, croscarmellose sodium NF, hydroxypropyl cellulose NF, magnesium stearate NF, hypromellose USP, titanium dioxide USP talc USP, polyethylene glycol NF, ferric oxide pigment, red NF, ferric oxide pigment, yellow NF.
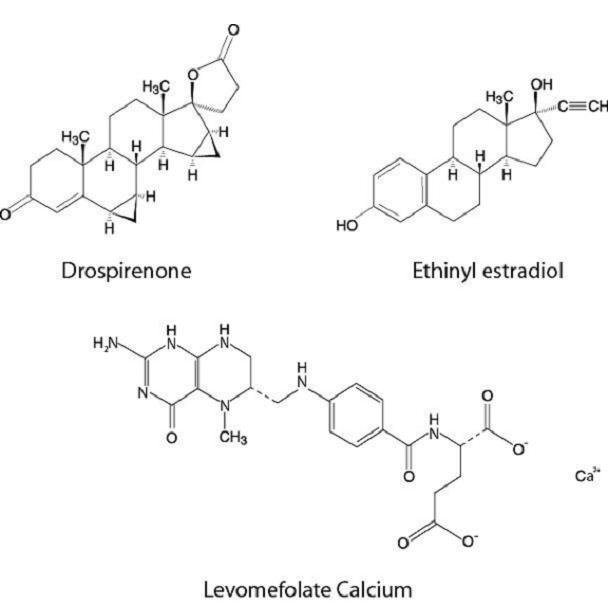
Drospirenone (6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-1,3',4',6,6a,7,8,9,10,11,12,13,14,15,15a,16-hexadecahydro-10,13-dimethylspiro-[17H-dicyclopropa- [6,7:15,16] cyclopenta[a]phenanthrene-17,2'(5H)-furan]-3,5'(2H)-dione) is a synthetic progestational compound and has a molecular weight of 366.5 and a molecular formula of C24H30O3.
Ethinyl estradiol (19-nor-17α-pregna 1,3,5(10)-triene-20-yne-3, 17-diol) is a synthetic estrogenic compound and has a molecular weight of 296.4 and a molecular formula of C20H24O2.
Levomefolate calcium (N-[4-[[(2-amino-1,4,5,6,7,8-hexahydro-5-methyl-4-oxo-(6S)-pteridinyl)methyl]amino]benzoyl]-L-glutamic acid, calcium salt) is a synthetic calcium salt of L-5-methyltetrahydrofolate (L-5-methyl-THF), which is a metabolite of vitamin B9 and has a molecular weight of 497.5 and a molecular formula of C20H23CaN7O6.
The structural formulas are as follows:
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - COCs lower the risk of becoming pregnant primarily by suppressing ovulation. 12.2 Pharmacodynamics - Drospirenone is a spironolactone analogue ...
12.1 Mechanism of Action
COCs lower the risk of becoming pregnant primarily by suppressing ovulation.
12.2 Pharmacodynamics
Drospirenone is a spironolactone analogue with anti-mineralocorticoid and antiandrogenic activity. The estrogen in Beyaz is ethinyl estradiol (EE).
Contraception
No specific pharmacodynamic studies were conducted with Beyaz.
Two studies evaluated the effect of 3 mg DRSP / 0.02 mg EE combinations on the suppression of ovarian activity as assessed by measurement of follicle size via transvaginal ultrasound and serum hormone (progesterone and estradiol) analyses during two treatment cycles (21-day active tablet period plus 7-day pill-free period). More than 90% of subjects in these studies demonstrated ovulation inhibition. One study compared the effect of 3 mg DRSP/0.02 mg EE combinations with two different regimens (24-day active tablet period plus 4-day pill-free period vs. 21-day active tablet period plus 7-day pill-free period) on the suppression of ovarian activity during two treatment cycles. During the first treatment cycle, there were no subjects (0/49, 0%) taking the 24-day regimen who ovulated compared to 1 subject (1/50, 2%) using the 21-day regimen. After intentionally introduced dosing errors (3 missed active tablets on Days 1 to 3) during the second treatment cycle, there was 1 subject (1/49, 2%) taking the 24-day regimen who ovulated compared to 4 subjects (4/50, 8%) using the 21-day regimen.
Acne
Acne vulgaris is a skin condition with a multifactorial etiology including androgen stimulation of sebum production. While the combination of EE and DRSP increases sex hormone binding globulin (SHBG) and decreases free testosterone, the relationship between these changes and a decrease in the severity of facial acne in otherwise healthy women with this skin condition has not been established. The impact of the antiandrogenic activity of DRSP on acne is not known.
Folate Supplementation
Two studies evaluated the impact of Beyaz on plasma folate and RBC folate levels. A randomized, double-blind, active-controlled, parallel group study compared plasma folate and red blood cell (RBC) folate levels during a 24-week treatment with YAZ + 0.451 mg levomefolate calcium as compared to YAZ alone in a U.S. population. The pharmacodynamic effect on plasma folate, RBC folate, and the profile of circulating folate metabolites was assessed during 24 weeks of treatment with 0.451 mg levomefolate calcium or with 0.4 mg folic acid (equimolar dose to 0.451 mg levomefolate calcium), both in combination with 3 mg DRSP/0.03 mg EE (Yasmin) followed by 20 weeks of open-label treatment with Yasmin only (elimination phase). [See Clinical Studies (14.4).]
Close12.3 Pharmacokinetics
Absorption
Beyaz and YAZ are bioequivalent with respect to DRSP and EE.
The absolute bioavailability of DRSP from a single entity tablet is about 76%. The absolute bioavailability of EE is approximately 40% as a result of presystemic conjugation and first-pass metabolism. The absolute bioavailability of Beyaz, which is a combination tablet of DRSP and EE stabilized by betadex as a clathrate (molecular inclusion complex), has not been evaluated. The bioavailability of EE is similar when dosed via a betadex clathrate formulation compared to when it is dosed as a free steroid. Serum concentrations of DRSP and EE reached peak levels within 1-2 hours after administration of Beyaz.
The pharmacokinetics of DRSP are dose proportional following single doses ranging from 1-10 mg. Following daily dosing of YAZ, steady state DRSP concentrations were observed after 8 days. There was about 2 to 3 fold accumulation in serum Cmax and AUC (0-24h) values of DRSP following multiple dose administration of YAZ (see Table 3).
For EE, steady-state conditions are reported during the second half of a treatment cycle. Following daily administration of YAZ, serum Cmax and AUC (0-24h) values of EE accumulate by a factor of about 1.5 to 2 (see Table 3).
Levomefolate calcium is structurally identical to L-5-methyltetrahydrofolate (L-5-methyl-THF), a metabolite of vitamin B9. Mean baseline concentrations of about 15 nmol/L are reached in populations without folate food fortification under normal nutritional conditions. Orally administered levomefolate calcium is absorbed and incorporated into the body folate pool. Peak plasma concentrations of about 50 nmol/L above baseline are reached within 0.5 – 1.5 hours after single oral administration of 0.451 mg levomefolate calcium.
Steady state conditions for total folate in plasma after intake of 0.451 mg levomefolate calcium are reached after about 8-16 weeks depending on the baseline levels. In red blood cells achievement of steady state is delayed due to the long life-span of red blood cells of about 120 days.
Table 3: Pharmacokinetic Parameters of YAZ (DRSP 3 mg and EE 0.02 mg) DRSP
Cycle / Day
No. of Subjects
Cmax*
(ng/mL)Tmax†
(h)AUC(0-24h)*
(ng•h/mL)t1/2*
(h)1/1
23
38.4 (25)
1.5 (1-2)
268 (19)
NA‡
1/21
23
70.3 (15)
1.5 (1-2)
763 (17)
30.8 (22)
EE
Cycle / Day
No. of Subjects
Cmax*
(pg/mL)Tmax†
(h)AUC(0-24h)*
(pg•h/mL)t1/2*
(h)1/1
23
32.8 (45)
1.5 (1-2)
108 (52)
NA‡
1/21
23
45.1 (35)
1.5 (1-2)
220 (57)
NA‡
Food Effect
The rate of absorption of DRSP and EE following single administration of a formulation similar to Beyaz was slower under fed (high fat meal) conditions with the serum Cmax being reduced about 40% for both components. The extent of absorption of DRSP, however, remained unchanged. In contrast, the extent of absorption of EE was reduced by about 20% under fed conditions.
The effect of food on absorption of levomefolate calcium following administration of Beyaz has not been evaluated.
Distribution
DRSP and EE serum concentrations decline in two phases. The apparent volume of distribution of DRSP is approximately 4 L/kg and that of EE is reported to be approximately 4–5 L/kg.
DRSP does not bind to sex hormone binding globulin (SHBG) or corticosteroid binding globulin (CBG) but binds about 97% to other serum proteins. Multiple dosing over 3 cycles resulted in no change in the free fraction (as measured at trough concentrations). EE is reported to be highly but non-specifically bound to serum albumin (approximately 98.5 %) and induces an increase in the serum concentrations of both SHBG and CBG. EE induced effects on SHBG and CBG were not affected by variation of the DRSP dosage in the range of 2 to 3 mg.
Biphasic kinetics is reported for folates with a fast- and a slow-turnover pool. The fast turnover pool, probably reflecting newly absorbed folate, is consistent with the terminal half-life of approximately 4 – 5 hours after single oral administration of 0.451 mg levomefolate calcium. The slow-turnover pool reflecting turnover of folate polyglutamate has a mean residence time of greater than or equal to 100 days.
Metabolism
The two main metabolites of DRSP found in human plasma were identified to be the acid form of DRSP generated by opening of the lactone ring and the 4,5-dihydrodrospirenone-3-sulfate, formed by reduction and subsequent sulfatation. These metabolites were shown not to be pharmacologically active. Drospirenone is also subject to oxidative metabolism catalyzed by CYP3A4.
EE has been reported to be subject to significant gut and hepatic first-pass metabolism. Metabolism of EE and its oxidative metabolites occur primarily by conjugation with glucuronide or sulfate. CYP3A4 in the liver is responsible for the 2-hydroxylation which is the major oxidative reaction. The 2-hydroxy metabolite is further transformed by methylation and glucuronidation prior to urinary and fecal excretion.
L-5-methyl-THF is the predominant folate transport form in blood under physiological conditions and during folic acid and levomefolate calcium administration.
Excretion
DRSP serum concentrations are characterized by a terminal disposition phase half-life of approximately 30 hours after both single and multiple dose regimens. Excretion of DRSP was nearly complete after ten days and amounts excreted were slightly higher in feces compared to urine. DRSP was extensively metabolized and only trace amounts of unchanged DRSP were excreted in urine and feces. At least 20 different metabolites were observed in urine and feces. About 38-47% of the metabolites in urine were glucuronide and sulfate conjugates. In feces, about 17-20% of the metabolites were excreted as glucuronides and sulfates.
For EE the terminal disposition phase half-life has been reported to be approximately 24 hours. EE is not excreted unchanged. EE is excreted in the urine and feces as glucuronide and sulfate conjugates and undergoes enterohepatic circulation.
L-5-methyl-THF is eliminated from the body by urinary excretion of intact folates and catabolic products as well as fecal excretion through a biphasic kinetics process.
Use in Specific Populations
Pediatric Use: Safety and efficacy of Beyaz has been established in women of reproductive age. Efficacy is expected to be the same for postpubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
Geriatric Use: Beyaz has not been studied in postmenopausal women and is not indicated in this population.
Race: No clinically significant difference was observed between the pharmacokinetics of DRSP or EE in Japanese versus Caucasian women (age 25-35) when 3 mg DRSP/0.02 mg EE was administered daily for 21 days. Other ethnic groups have not been specifically studied.
Renal Impairment: Beyaz is contraindicated in patients with renal impairment.
The effect of renal impairment on the pharmacokinetics of DRSP (3 mg daily for 14 days) and the effect of DRSP on serum potassium concentrations were investigated in three separate groups of female subjects (n=28, age 30-65). All subjects were on a low potassium diet. During the study, 7 subjects continued the use of potassium-sparing drugs for the treatment of their underlying illness. On the 14th day (steady-state) of DRSP treatment, the serum DRSP concentrations in the group with CLcr of 50–79 mL/min were comparable to those in the control group with CLcr ≥ 80 mL/min. The serum DRSP concentrations were on average 37% higher in the group with CLcr of 30–49 mL/min compared to those in the control group. DRSP treatment did not show any clinically significant effect on serum potassium concentration. Although hyperkalemia was not observed in the study, in five of the seven subjects who continued use of potassium-sparing drugs during the study, mean serum potassium concentrations increased by up to 0.33 mEq/L. [See Contraindications (4), and Warnings and Precautions (5.2).]
Hepatic Impairment: Beyaz is contraindicated in patients with hepatic disease.
The mean exposure to DRSP in women with moderate liver impairment is approximately three times higher than the exposure in women with normal liver function. Beyaz has not been studied in women with severe hepatic impairment. [See Contraindications (4) and Warnings and Precautions (5.4).]
Drug Interactions
Consult the labeling of all concurrently used drugs to obtain further information about interactions with oral contraceptives or the potential for enzyme alterations.
Effects of Other Drugs on Combined Oral Contraceptives
Substances diminishing the efficacy of COCs: Drugs or herbal products that induce certain enzymes, including CYP3A4, may decrease the effectiveness of COCs or increase breakthrough bleeding.
Substances increasing the plasma concentrations of COCs: Co-administration of atorvastatin and certain COCs containing EE increase AUC values for EE by approximately 20%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation. In a clinical drug-drug interaction study conducted in 20 premenopausal women, co-administration of a DRSP (3 mg)/EE (0.02 mg) COC with the strong CYP3A4 inhibitor ketoconazole (200 mg twice daily) for 10 days increased the AUC(0-24h) of DRSP and EE by 2.68-fold (90% CI: 2.44, 2.95) and 1.40-fold (90% CI: 1.31, 1.49), respectively. The increases in Cmax were 1.97-fold (90% CI: 1.79, 2.17) and 1.39-fold (90% CI: 1.28, 1.52) for DRSP and EE, respectively. Although no clinically relevant effects on safety or laboratory parameters including serum potassium were observed, this study only assessed subjects for 10 days. The clinical impact for a patient taking a DRSP-containing COC concomitantly with chronic use of a CYP3A4/5 inhibitor is unknown [see Warnings and Precautions (5.2)].
HIV/HCV protease inhibitors and non-nucleoside reverse transcriptase inhibitors: Significant changes (increase or decrease) in the plasma concentrations of estrogen and progestin have been noted in some cases of co-administration with HIV/HCV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Effects of Combined Oral Contraceptives on Other Drugs
COCs containing EE may inhibit the metabolism of other compounds. COCs have been shown to significantly decrease plasma concentrations of lamotrigine, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary. Consult the labeling of the concurrently-used drug to obtain further information about interactions with COCs or the potential for enzyme alterations.
In vitro, EE is a reversible inhibitor of CYP2C19, CYP1A1 and CYP1A2 as well as a mechanism-based inhibitor of CYP3A4/5, CYP2C8, and CYP2J2. Metabolism of DRSP and potential effects of DRSP on hepatic CYP enzymes have been investigated in in vitro and in vivo studies. In in vitro studies DRSP did not affect turnover of model substrates of CYP1A2 and CYP2D6, but had an inhibitory influence on the turnover of model substrates of CYP1A1, CYP2C9, CYP2C19, and CYP3A4, with CYP2C19 being the most sensitive enzyme. The potential effect of DRSP on CYP2C19 activity was investigated in a clinical pharmacokinetic study using omeprazole as a marker substrate. In the study with 24 postmenopausal women [including 12 women with homozygous (wild type) CYP2C19 genotype and 12 women with heterozygous CYP2C19 genotype] the daily oral administration of 3 mg DRSP for 14 days did not affect the oral clearance of omeprazole (40 mg, single oral dose) and the CYP2C19 product 5-hydroxy omeprazole. Furthermore, no significant effect of DRSP on the systemic clearance of the CYP3A4 product omeprazole sulfone was found. These results demonstrate that DRSP did not inhibit CYP2C19 and CYP3A4 in vivo.
Two additional clinical drug-drug interaction studies using simvastatin and midazolam as marker substrates for CYP3A4 were each performed in 24 healthy postmenopausal women. The results of these studies demonstrated that pharmacokinetics of the CYP3A4 substrates were not influenced by steady state DRSP concentrations achieved after administration of 3 mg DRSP/day.
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentration of thyroid-binding globulin increases with use of COCs.
Interactions With Drugs That Have the Potential to Increase Serum Potassium Concentration:
There is a potential for an increase in serum potassium concentration in women taking Beyaz with other drugs that may increase serum potassium concentration [see Warnings and Precautions (5.2)].
A drug-drug interaction study of DRSP 3 mg/estradiol (E2) 1 mg versus placebo was performed in 24 mildly hypertensive postmenopausal women taking enalapril maleate 10 mg twice daily. Potassium concentrations were obtained every other day for a total of 2 weeks in all subjects. Mean serum potassium concentrations in the DRSP/E2 treatment group relative to baseline were 0.22 mEq/L higher than those in the placebo group. Serum potassium concentrations also were measured at multiple time points over 24 hours at baseline and on Day 14. On Day 14, the ratios for serum potassium Cmax and AUC in the DRSP/E2 group to those in the placebo group were 0.955 (90% CI: 0.914, 0.999) and 1.010 (90% CI: 0.944, 1.08), respectively. No patient in either treatment group developed hyperkalemia (serum potassium concentrations >5.5 mEq/L).
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 24 month oral carcinogenicity study in mice dosed with 10 mg/kg/day DRSP alone or 1 + 0.01, 3 + 0.03 and 10 + 0.1 mg/kg/day of ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24 month oral carcinogenicity study in mice dosed with 10 mg/kg/day DRSP alone or 1 + 0.01, 3 + 0.03 and 10 + 0.1 mg/kg/day of DRSP and EE, 0.1 to 2 times the exposure (AUC of DRSP) of women taking a contraceptive dose, there was an increase in carcinomas of the harderian gland in the group that received the high dose of DRSP alone. In a similar study in rats given 10 mg/kg/day DRSP alone or 0.3 + 0.003, 3 + 0.03 and 10 + 0.1 mg/kg/day DRSP and EE, 0.8 to 10 times the exposure of women taking a contraceptive dose, there was an increased incidence of benign and total (benign and malignant) adrenal gland pheochromocytomas in the group receiving the high dose of DRSP. Mutagenesis studies for DRSP were conducted in vivo and in vitro and no evidence of mutagenic activity was observed. [see Warnings and Precautions (5.3), (5.4)].
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of levomefolate. Mutagenesis studies for levomefolate were conducted in vitro and in vivo and no evidence of mutagenic activity was observed.
-
14 CLINICAL STUDIES14.1 Oral Contraceptive Clinical Trial - In the primary contraceptive efficacy study of YAZ (3 mg DRSP/0.02 mg EE) of up to 1 year duration, 1,027 subjects were enrolled and completed 11,480 ...
14.1 Oral Contraceptive Clinical Trial
In the primary contraceptive efficacy study of YAZ (3 mg DRSP/0.02 mg EE) of up to 1 year duration, 1,027 subjects were enrolled and completed 11,480 28-day cycles of use. The age range was 17 to 36 years. The racial demographic was: 87.8% Caucasian, 4.6% Hispanic, 4.3% Black, 1.2% Asian, and 2.1% other. Women with a BMI greater than 35 were excluded from the trial. The pregnancy rate (Pearl Index) was 1.41 (95% CI [0.73 – 2.47]) per 100 woman-years of use based on 12 pregnancies that occurred after the onset of treatment and within 14 days after the last dose of YAZ in women 35 years of age or younger during cycles in which no other form of contraception was used.
14.2 Premenstrual Dysphoric Disorder Clinical Trials
Two multicenter, double-blind, randomized, placebo-controlled studies were conducted to evaluate the effectiveness of YAZ in treating the symptoms of PMDD. Women aged 18-42 who met DSM-IV criteria for PMDD, confirmed by prospective daily ratings of their symptoms, were enrolled. Both studies measured the treatment effect of YAZ using the Daily Record of Severity of Problems scale, a patient-rated instrument that assesses the symptoms that constitute the DSM-IV diagnostic criteria. The primary study was a parallel group design that included 384 evaluable reproductive-aged women with PMDD who were randomly assigned to receive YAZ or placebo treatment for 3 menstrual cycles. The supportive study, a crossover design, was terminated prematurely prior to achieving recruitment goals due to enrollment difficulties. A total of 64 women of reproductive age with PMDD were treated initially with YAZ or placebo for up to 3 cycles followed by a washout cycle and then crossed over to the alternate medication for 3 cycles.
Efficacy was assessed in both studies by the change from baseline during treatment using a scoring system based on the first 21 items of the Daily Record of Severity of Problems. Each of the 21 items was rated on a scale from 1 (not at all) to 6 (extreme); thus a maximum score of 126 was possible. In both trials, women who received YAZ had statistically significantly greater improvement in their Daily Record of Severity of Problems scores. In the primary study, the average decrease (improvement) from baseline was 37.5 points in women taking YAZ, compared to 30.0 points in women taking placebo.
14.3 Acne Clinical Trials
In two multicenter, double-blind, randomized, placebo-controlled studies, 889 subjects, ages 14 to 45 years, with moderate acne received YAZ or placebo for six 28-day cycles. The primary efficacy endpoints were the percent change in inflammatory lesions, non-inflammatory lesions, total lesions, and the percentage of subjects with a "clear" or "almost clear" rating on the Investigator's Static Global Assessment (ISGA) scale on day 15 of cycle 6, as presented in Table 4:
Table 4: Efficacy Results for Acne Trials* - *
- Evaluated at day 15 of cycle 6, last observation carried forward for the Intent to treat population
Study 1
Study 2
YAZ
Placebo
YAZ
Placebo
N=228
N=230
N=218
N=213
ISGA Success Rate
35 (15%)
10 (4%)
46 (21%)
19 (9%)
Inflammatory Lesions
Mean Baseline Count
Mean Absolute (%) Reduction
33
15 (48%)
33
11 (32%)
32
16 (51%)
32
11 (34%)
Non-inflammatory Lesions
Mean Baseline Count
Mean Absolute (%) Reduction
47
18 (39%)
47
10 (18%)
44
17 (42%)
44
11 (26%)
Total Lesions
Mean Baseline Count
Mean Absolute (%) Reduction
80
33 (42%)
80
21 (25%)
76
33 (46%)
76
22 (31%)
Close14.4 Folate Supplementation Clinical Trials
The development program for Beyaz consisted of two clinical trials.
One study was a multicenter, randomized, double-blind, active-controlled, parallel group US study. Plasma folate and red blood cell folate levels were investigated during a 24-week treatment with YAZ + 0.451 mg levomefolate calcium as compared to YAZ alone in a U.S. population with folate fortified food. A total of 379 healthy women between 18 and 40 years of age with no restrictions on folate supplementation received YAZ + levomefolate calcium (N= 285) or YAZ (N=94). The plasma and RBC folate concentrations at Week 24 were the co-primary endpoints. Figures 4 and 5 display the results for plasma and RBC folate, respectively, among evaluable subjects in each arm of the study.
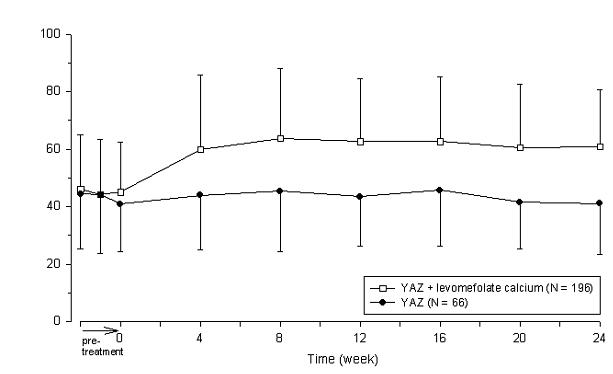
Figure 4: US Study: Mean concentration-time curves (and SD) of plasma folates after daily oral administration of YAZ + levomefolate calcium and YAZ
Arithmetic mean values based on 4-weekly measurements are displayed with arithmetic standard deviations which are shown in only one direction to improve readability. Data are based on per protocol analysis populations. The SD bar shown represents a single SD.
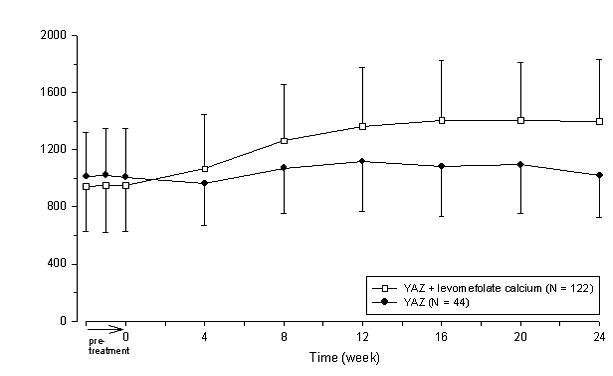
Figure 5: US Study Mean concentration-time curves (and SD) of RBC folates after daily oral administration of YAZ + levomefolate calcium and YAZ
Arithmetic mean values based on 4-weekly measurements are displayed with arithmetic standard deviations which are shown in only one direction to improve readability. Data are based on per protocol analysis populations. The SD bar shown represents a single SD.
In the second study, the pharmacodynamic effect on plasma folate, RBC folate, and the profile of circulating folate metabolites was assessed during 24 weeks of treatment with 0.451 mg levomefolate calcium or with 0.4 mg folic acid (equimolar dose to 0.451 mg levomefolate calcium), both in combination with 3 mg DRSP/0.03 mg EE (Yasmin) followed by 20 weeks of open-label treatment with Yasmin only (elimination phase). One-hundred and seventy-two healthy women between 18 to 40 years of age from a German population without folate food fortification and without concomitant intake of folate supplements were randomized to one of the two treatments. Figures 6 and 7 display the results for plasma and RBC folate, respectively, among evaluable subjects in the levomefolate arm of the study.
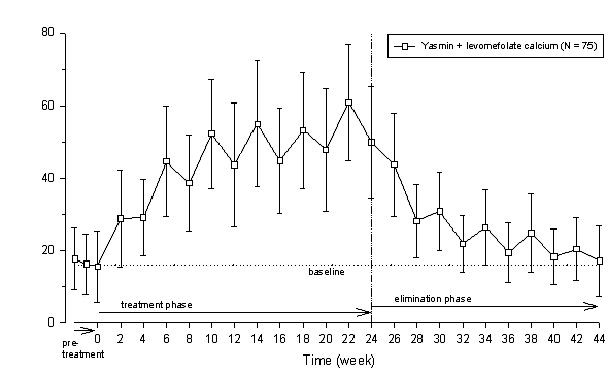
Figure 6: German Study: Mean trough concentration-time curve (and SD) of plasma folates after daily oral administration of Yasmin + levomefolate calcium
Arithmetic mean values based on biweekly measurements are displayed with arithmetic standard deviations. In the treatment phase, women received Yasmin + levomefolate calcium; in the elimination phase, all women received Yasmin only. Data are based on per protocol analysis populations. The SD bar shown represents a single SD.
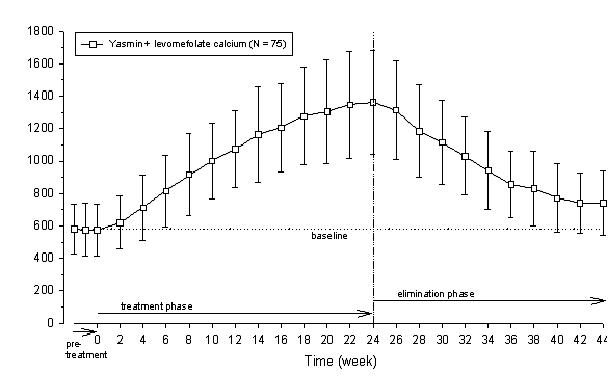
Figure 7: German Study: Mean concentration-time curves (and SD) of RBC folates after daily oral administration of Yasmin + levomefolate calcium
Arithmetic mean values based on biweekly measurements are displayed with arithmetic standard deviations. In the treatment phase, women received Yasmin + levomefolate calcium; in the elimination phase, all women received Yasmin only. Data are based on per protocol analysis populations. The SD bar shown represents a single SD.
The potential to reduce the incidence of neural tube defects (NTDs) with folate supplementation is well established based on a body of evidence derived from randomized, controlled trials, nonrandomized intervention trials, and observational studies using folic acid. Therefore, the Centers for Disease Control and Prevention (CDC) and the U.S. Preventive Services Task Force recommend that women of childbearing age consume supplemental folic acid in a dose of at least 0.4 mg (400 mcg) daily 1,6.
-
15 REFERENCES1. US Preventive Services Task Force. Folic Acid for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2009;150:626-631. 2. Seeger ...
1. US Preventive Services Task Force. Folic Acid for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. Ann Intern Med 2009;150:626-631.
2. Seeger, J.D., Loughlin, J., Eng, P.M., Clifford, C.R., Cutone, J., and Walker, A.M. (2007). Risk of thromboembolism in women taking ethinylestradiol/drospirenone and other oral contraceptives. Obstet Gynecol 110, 587-593.
3. Dinger, J.C., Heinemann, L.A., and Kuhl-Habich, D. (2007). The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 75, 344-354.
4. Combined hormonal contraceptives (CHCs) and the risk of cardiovascular endpoints. Sidney, S. (primary author) http://www.fda.gov/downloads/Drugs/DrugSafety/UCM277384.pdf, accessed Oct 27, 2011.
5. Lidegaard, O., Lokkegaard, E., Svendsen, A.L., and Agger, C. (2009). Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ 339, b2890.
6. Lidegaard, O., Nielsen, L.H., Skovlund, C.W., Skjeldestad, F.E., and Lokkegaard, E. (2011). Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ 343, d6423.
7. van Hylckama Vlieg, A., Helmerhorst, F.M., Vandenbroucke, J.P., Doggen, C.J., and Rosendaal, F.R. (2009). The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ 339, b2921.
8. Dinger, J., Assmann, A., Mohner, S., and Minh, T.D. (2010). Risk of venous thromboembolism and the use of dienogest- and drospirenone-containing oral contraceptives: results from a German case-control study. J Fam Plann Reprod Health Care 36, 123-129.
9. Jick, S.S., and Hernandez, R.K. (2011). Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 342, d2151.
10. Parkin, L., Sharples, K., Hernandez, R.K., and Jick, S.S. (2011). Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 342, d2139.
11. Centers for Disease Control. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR 1992;41(No. RR-14).
Close -
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Beyaz (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) are available in packages of three blister packs (NDC 50419-407-03). The ...
16.1 How Supplied
Beyaz (drospirenone/ethinyl estradiol/levomefolate calcium tablets and levomefolate calcium tablets) are available in packages of three blister packs (NDC 50419-407-03).
The film-coated tablets are rounded with biconvex faces, one side is embossed with a regular hexagon shape with Z+ or M+.
Each blister pack (28 film-coated tablets) contains in the following order:
- •
- 24 round, biconvex, pink, film-coated tablets with embossed “Z+” in a regular hexagon on one side each containing 3 mg drospirenone, 0.02 mg ethinyl estradiol, and 0.451 mg levomefolate calcium
- •
- 4 round, biconvex, light orange, film-coated tablets with embossed “M+” in a regular hexagon on one side each containing 0.451 mg levomefolate calcium
Close16.2 Storage
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). • Counsel patients that cigarette smoking increases the risk of serious cardiovascular events from COC use, and ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- •
- Counsel patients that cigarette smoking increases the risk of serious cardiovascular events from COC use, and that women who are over 35 years old and smoke should not use COCs.
- •
- Counsel patients that the increased risk of VTE compared to non-users of COCs is greatest after initially starting a COC or restarting (following a 4-week or greater pill-free interval) the same or a different COC.
- •
- Counsel patients about the information regarding the risk of VTE with DRSP-containing COCs compared to COCs that contain levonorgestrel or some other progestins.
- •
- Counsel patients that Beyaz does not protect against HIV-infection (AIDS) and other sexually transmitted diseases.
- •
- Counsel patients on Warnings and Precautions associated with COCs.
- •
- Counsel patients that Beyaz contains DRSP. Drospirenone may increase potassium. Patients should be advised to inform their healthcare provider if they have kidney, liver or adrenal disease because the use of Beyaz in the presence of these conditions could cause serious heart and health problems. They should also inform their healthcare provider if they are currently on daily, long-term treatment (NSAIDs, potassium-sparing diuretics, potassium supplementation, ACE inhibitors, angiotensin-II receptor antagonists, heparin or aldosterone antagonists) for a chronic condition or taking strong CYP3A4 inhibitors.
- •
- Inform patients that Beyaz is not indicated during pregnancy. If pregnancy occurs during treatment with Beyaz, instruct the patient to stop further intake. However, women should be advised on the continued need of sufficient folate intake.
- •
- Counsel patients to take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event pills are missed.
- •
- Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with COCs.
- •
- Counsel patients who are breastfeeding or who desire to breastfeed that COCs may reduce breast milk production. This is less likely to occur if breastfeeding is well established.
- •
- Counsel any patient who starts COCs postpartum, and who has not yet had a period, to use an additional method of contraception until she has taken a pink tablet for 7 consecutive days.
- •
- Counsel patients that amenorrhea may occur. Rule out pregnancy in the event of amenorrhea in two or more consecutive cycles.
- •
- Counsel patients to report whether they are taking folate supplements. Beyaz contains the equivalent of 0.4 mg (400 mcg) of folic acid.
- •
- Counsel patients to maintain folate supplementation if they discontinue Beyaz due to pregnancy.
-
FDA Approved Patient LabelingGuide for Using Beyaz - WARNING TO WOMEN WHO SMOKE - Do not use Beyaz if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects ...
Guide for Using Beyaz
WARNING TO WOMEN WHO SMOKE
Do not use Beyaz if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
CloseBirth control pills help to lower the chances of becoming pregnant when taken as directed. They do not protect against HIV infection (AIDS) and other sexually transmitted diseases.
What Is Beyaz?
Beyaz is a birth control pill. It contains two female hormones, a synthetic estrogen called ethinyl estradiol and a progestin called drospirenone. Beyaz also contains levomefolate calcium, which is a B vitamin.
The progestin drospirenone may increase potassium. Therefore, you should not take Beyaz if you have kidney, liver or adrenal disease because this could cause serious heart and health problems. Other drugs may also increase potassium. If you are currently on daily, long-term treatment for a chronic condition with any of the medications below, you should consult your healthcare provider about whether Beyaz is right for you, and during the first month that you take Beyaz, you should have a blood test to check your potassium level.
- •
- NSAIDs (ibuprofen [Motrin, Advil], naproxen [Aleve and others] when taken long-term and daily for treatment of arthritis or other problems)
- •
- Potassium-sparing diuretics (spironolactone and others)
- •
- Potassium supplementation
- •
- ACE inhibitors (Capoten, Vasotec, Zestril and others)
- •
- Angiotensin-II receptor antagonists (Cozaar, Diovan, Avapro and others)
- •
- Heparin
- •
- Aldosterone antagonists
Beyaz may also be taken to treat premenstrual dysphoric disorder (PMDD) if you choose to use the Pill for birth control. Unless you have already decided to use the Pill for birth control, you should not start Beyaz to treat your PMDD because there are other medical therapies for PMDD that do not have the same risks as the Pill. PMDD is a mood disorder related to the menstrual cycle. PMDD significantly interferes with work or school, or with usual social activities and relationships with others. Symptoms include markedly depressed mood, anxiety or tension, mood swings, and persistent anger or irritability. Other features include decreased interest in usual activities, difficulty concentrating, lack of energy, change in appetite or sleep, and feeling out of control. Physical symptoms associated with PMDD may include breast tenderness, headache, joint and muscle pain, bloating and weight gain. These symptoms occur regularly before menstruation starts and go away within a few days following the start of the period. Diagnosis of PMDD should be made by healthcare providers.
You should only use Beyaz for treatment of PMDD if you:
- •
- Have already decided to use oral contraceptives for birth control, and
- •
- Have been diagnosed with PMDD by your healthcare provider.
Beyaz has not been shown to be effective for the treatment of premenstrual syndrome (PMS), a less serious set of symptoms occurring before menstruation. If you or your healthcare provider believe you have PMS, you should take Beyaz only if you want to prevent pregnancy; and not for the treatment of PMS.
Beyaz may also be taken to treat moderate acne if all of the following are true:
- •
- Your healthcare provider says it is safe for you to use Beyaz.
- •
- You are at least 14 years old.
- •
- You have started having menstrual periods.
- •
- You want to use a birth control pill to prevent pregnancy.
Beyaz may also be taken to provide folate supplementation in women who elect to use an oral contraceptive. It is recommended that women of reproductive age supplement their diet with 0.4 mg (400 mcg) of folic acid daily to lower their risk of having a pregnancy with a rare type of birth defect (known as a neural tube defect). The amount of folate contained in Beyaz supplements folate in the diet to lower this risk should you become pregnant while taking the drug or shortly after stopping it.
How Well Does Beyaz Work?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
Based on the results of one clinical study, 1 to 2 women out of 100 women, may get pregnant during the first year they use Beyaz.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
How Do I Take Beyaz?
1. Be sure to read these directions before you start taking your pills or anytime you are not sure what to do.
2. The right way to take the pill is to take one pill every day at the same time in the order directed on the package. Preferably, take the pill after the evening meal or at bedtime, with some liquid, as needed. Beyaz can be taken without regard to meals.
If you miss pills you could get pregnant. This includes starting the pack late. The more pills you miss, the more likely you are to get pregnant. See "WHAT TO DO IF YOU MISS PILLS” below.
3. Many women have spotting or light bleeding at unexpected times, or may feel sick to their stomach during the first 1-3 packs of pills.
If you do have spotting or light bleeding or feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If it does not go away, check with your healthcare provider.
4. Missing pills can also cause spotting or light bleeding, even when you make up these missed pills.
On the days you take two pills, to make up for missed pills, you could also feel a little sick to your stomach.
5. If you have vomiting (within 3 to 4 hours after you take your pill), you should follow the instructions for "WHAT TO DO IF YOU MISS PILLS." If you have diarrhea or if you take certain medicines, including some antibiotics and some herbal products such as St. John's Wort, your pills may not work as well.
Use a back-up method (such as condoms and spermicides) until you check with your healthcare provider.
6. If you have trouble remembering to take the pill, talk to your healthcare provider about how to make pill-taking easier or about using another method of birth control.
7. If you have any questions or are unsure about the information in this leaflet, call your healthcare provider.
Before You Start Taking Your Pills
1. Decide What Time of Day You Want to Take Your Pill
It is important to take Beyaz in the order directed on the package at the same time every day, preferably after the evening meal or at bedtime, with some liquid, as needed. Beyaz can be taken without regard to meals.
2. Look at Your Pill Pack—It has 28 Pills
The Beyaz-pill pack has 24 pink pills (with hormones and folate) to be taken for 24 days, followed by 4 light orange pills (without hormones, containing folate) to be taken for the next four days. It is important to take the light orange pills because they contain folate.
3. Also look for:
a) Where on the pack to start taking pills,
b) In what order to take the pills (follow the arrows)
4. Be sure you have ready at all times (a) another kind of birth control (such as condoms and spermicides) to use as a back-up in case you miss pills, and (b) an extra, full pill pack.
When To Start the First Pack of Pills
You have a choice for which day to start taking your first pack of pills. Decide with your healthcare provider which is the best day for you. Pick a time of day which will be easy to remember.
Day 1 Start:
1. Take the first pink pill of the pack during the first 24 hours of your period.
2. You will not need to use a back-up method of birth control, since you are starting the Pill at the beginning of your period. However, if you start Beyaz later than the first day of your period, you should use another method of birth control (such as a condom and spermicide) as a back-up method until you have taken 7 pink pills.
Sunday Start:
1. Take the first pink pill of the pack on the Sunday after your period starts, even if you are still bleeding. If your period begins on Sunday, start the pack that same day.
2. Use another method of birth control (such as a condom and spermicide) as a back-up method if you have sex anytime from the Sunday you start your first pack until the next Sunday (7 days). This also applies if you start Beyaz after having been pregnant, and you have not had a period since your pregnancy.
When You Switch From a Different Birth Control Pill
When switching from another birth control pill, Beyaz should be started on the same day that a new pack of the previous birth control pill would have been started.
When You Switch From Another Type of Birth Control Method
When switching from a transdermal patch or vaginal ring, Beyaz should be started when the next application would have been due. When switching from an injection, Beyaz should be started when the next dose would have been due. When switching from an intrauterine contraceptive or an implant, Beyaz should be started on the day of removal.
What to Do During the Month
1. Take one pill at the same time every day until the pack is empty.
Do not skip pills even if you are spotting or bleeding between monthly periods or feel sick to your stomach (nausea).
Do not skip pills even if you do not have sex very often.
2. When you finish a pack of pills, start the next pack on the day after your last light orange pill. It is important to take the light orange pills because they contain folate. Do not wait any days between packs.
What to Do if You Miss Pills
If you miss 1 pink pill in your pack:
1. Take it as soon as you remember. Take the next pill at your regular time. This means you may take two pills in one day.
2. You do not need to use a back-up birth control method if you have sex.
If you miss 2 pink pills in a row in Week 1 or Week 2 of your pack:
1. Take two pills on the day you remember and two pills the next day.
2. Then take one pill a day until you finish the pack.
3. You could become pregnant if you have sex in the 7 days after you restart your pills. You must use another birth control method (such as a condom and spermicide) as a back-up for those 7 days.
If you miss 2 pink pills in a row in Week 3 or Week 4 of your pack:
1. If you are a Day 1 Starter:
Throw out the rest of the pill pack and start a new pack that same day.
If you are a Sunday Starter:
Keep taking one pill every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of pills that same day.
2. You could become pregnant if you have sex in the 7 days after you restart your pills. You must use another birth control method (such as a condom and spermicide) as a back-up for those 7 days.
3. You may not have your period this month but this is expected. However, if you miss your period two months in a row, call your healthcare provider because you might be pregnant.
If you miss 3 or more pink pills in a row during any week:
1. If you are a Day 1 Starter:
Throw out the rest of the pill pack and start a new pack that same day.
If you are a Sunday Starter:
Keep taking 1 pill every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of pills that same day.
2. You could become pregnant if you have sex in the 7 days after you restart your pills. You must use another birth control method (such as condoms and spermicides) as a back-up for those 7 days.
3. You may not have your period this month but this is expected. However, if you miss your period two months in a row, call your healthcare provider because you might be pregnant.
If you miss any of the 4 light orange pills in Week 4:
Throw away the pills you missed.
Keep taking one pill each day until the pack is empty.
You do not need a back-up method.
Finally, if you are still not sure what to do about the pills you have missed:
Use a back-up method (such as condoms and spermicides) anytime you have sex.
Contact your healthcare provider and continue taking one active pink pill each day until otherwise directed.
WHO SHOULD NOT TAKE Beyaz?
Your healthcare provider will not give you Beyaz if you:
- •
- Ever had blood clots in your legs (deep vein thrombosis), lungs (pulmonary embolism), or eyes (retinal thrombosis)
- •
- Ever had a stroke
- •
- Ever had a heart attack
- •
- Have certain heart valve problems or heart rhythm abnormalities that can cause blood clots to form in the heart
- •
- Have an inherited problem with your blood that makes it clot more than normal
- •
- Have high blood pressure that medicine can't control
- •
- Have diabetes with kidney, eye, nerve, or blood vessel damage
- •
- Ever had certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision
- •
- Ever had breast cancer which may be sensitive to female hormones
- •
- Have liver disease, including liver tumors
- •
- Take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood.
- •
- Have kidney disease
- •
- Have adrenal disease
Also, do not take birth control pills if you:
- •
- Smoke and are over 35 years old
- •
- Are or suspect you are pregnant
Birth control pills may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy (also called cholestasis of pregnancy).
Tell your healthcare provider if you have ever had any of the above conditions (your healthcare provider can recommend another method of birth control).
Tell your healthcare provider if you are already taking daily folate supplements.
What Else Should I Know about Taking Beyaz?
Birth control pills do not protect you against any sexually transmitted disease, including HIV, the virus that causes AIDS.
Do not skip any pills, even if you do not have sex often.
If you miss a period, you could be pregnant. However, some women miss periods or have light periods on birth control pills, even when they are not pregnant. Contact your healthcare provider for advice if you:
- •
- Think you are pregnant
- •
- Miss one period and have not taken your birth control pills every day
- •
- Miss two periods in a row
Birth control pills should not be taken during pregnancy. However, birth control pills taken by accident during pregnancy are not known to cause birth defects.
You should stop Beyaz at least four weeks before you have major surgery and not restart it until at least two weeks after the surgery due to an increased risk of blood clots.
If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like Beyaz, may decrease the amount of milk you make. A small amount of the pill's hormones pass into breast milk.
Folates may make certain drugs, including some used for epilepsy, less effective, so talk to your healthcare provider about any medicines you take.
If you have vomiting or diarrhea, your birth control pills may not work as well. Use another birth control method, like condoms and a spermicide, until you check with your healthcare provider.
If you are scheduled for any laboratory tests, tell your doctor you are taking birth-control pills. Certain blood tests may be affected by birth-control pills.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Beyaz may affect the way other medicines work, and other medicines may affect how well Beyaz works. Know the medicines you take.
Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
What are the Most Serious Risks of Taking Birth Control Pills?
Like pregnancy, birth control pills increase the risk of serious blood clots (see following graph), especially in women who have other risk factors, such as smoking, obesity, or age greater than 35. This increased risk is highest when you first start taking birth control pills and when you restart the same or different birth control pills after not using them for a month or more. Women who use birth control pills with drospirenone (like Beyaz) may have a higher risk of getting a blood clot. Some studies reported that the risk of blood clots was higher for women who use birth control pills that contain drospirenone than for women who use birth control pills that do not contain drospirenone.
Talk with your healthcare provider about your risk of getting a blood clot before deciding which birth control pill is right for you.
It is possible to die or be permanently disabled from a problem caused by a blood clot, such as a heart attack or a stroke.
Some examples of serious clots are blood clots in the:
- •
- Legs (deep vein thrombosis or DVT)
- •
- Lungs (pulmonary embolus or PE)
- •
- Eyes (loss of eyesight)
- •
- Heart (heart attack)
- •
- Brain (stroke)
To put the risk of developing a blood clot into perspective: If 10,000 women who are not pregnant and do not use birth control pills are followed for one year, between 1 and 5 of these women will develop a blood clot. The figure below shows the likelihood of developing a serious blood clot for women who are not pregnant and do not use birth control pills, for women who use birth control pills, for pregnant women, and for women in the first 12 weeks after delivering a baby.
Likelihood of Developing a Serious Blood Clot
A few women who take birth control pills may get:
- •
- High blood pressure
- •
- Gallbladder problems
- •
- Rare cancerous or noncancerous liver tumors
- All of these events are uncommon in healthy women.
Call your healthcare provider right away if you have:
- •
- Persistent leg pain
- •
- Sudden shortness of breath
- •
- Sudden blindness, partial or complete
- •
- Severe pain in your chest
- •
- Sudden, severe headache unlike your usual headaches
- •
- Weakness or numbness in an arm or leg, or trouble speaking
- •
- Yellowing of the skin or eyeballs
What are the Common Side Effects of Birth Control Pills?
The most common side effects of birth control pills are:
- •
- Spotting or bleeding between menstrual periods
- •
- Nausea
- •
- Breast tenderness
- •
- Headache
These side effects are usually mild and usually disappear with time.
Less common side effects are:
- •
- Acne
- •
- Less sexual desire
- •
- Bloating or fluid retention
- •
- Blotchy darkening of the skin, especially on the face
- •
- High blood sugar, especially in women who already have diabetes
- •
- High fat (cholesterol; triglyceride) levels in the blood
- •
- Depression, especially if you have had depression in the past. Call your healthcare provider immediately if you have any thoughts of harming yourself.
- •
- Problems tolerating contact lenses
- •
- Weight changes
This is not a complete list of possible side effects. Talk to your healthcare provider if you develop any side effects that concern you. You may report side effects to the FDA at 1-800-FDA-1088.
No serious problems have been reported from a birth control pill overdose, even when accidentally taken by children.
Do Birth Control Pills Cause Cancer?
It is not known if hormonal birth control pills cause breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
Women who use birth control pills may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What Should I Know about My Period when Taking Beyaz?
Irregular vaginal bleeding or spotting may occur while you are taking Beyaz. Irregular bleeding may vary from slight staining between menstrual periods to breakthrough bleeding, which is a flow much like a regular period. Irregular bleeding occurs most often during the first few months of oral contraceptive use, but may also occur after you have been taking the pill for some time. Such bleeding may be temporary and usually does not indicate any serious problems. It is important to continue taking your pills on schedule. If the bleeding occurs in more than one cycle, is unusually heavy, or lasts for more than a few days, call your healthcare provider.
Some women may not have a menstrual period but this should not be cause for alarm as long as you have taken the pills according to direction.
What if I Miss My Scheduled Period when Taking Beyaz?
It is not uncommon to miss your period. However, if you miss two periods in a row or miss one period when you have not taken your birth control pills according to directions, call your healthcare provider. Also notify your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. It is important that your healthcare provider checks you to find out if you are pregnant. Stop taking Beyaz if you are pregnant.
What If I Want to Become Pregnant?
You may stop taking the pill whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking the pill. See your health care provider about appropriate folate supplementation if you stop taking Beyaz, are pregnant, or plan on becoming pregnant.
General Advice about Beyaz
Your healthcare provider prescribed Beyaz for you. Please do not share Beyaz with anyone else. Keep Beyaz out of the reach of children.
If you have concerns or questions, ask your healthcare provider. You may also ask your healthcare provider for a more detailed label written for medical professionals.
Revised: 5/2023
Manufactured for:
Bayer HealthCare Pharmaceuticals Inc.
Whippany, NJ 07981Manufactured in Germany
©2010 Bayer HealthCare Pharmaceuticals Inc. All Rights Reserved
Bayer HealthCare Pharmaceuticals Inc.
-
PRINCIPAL DISPLAY PANELBeyaz Trade Blisterpack - NDC 50419-407-01 - 1 Unit - Rx only - This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other ...
Beyaz Trade Blisterpack
NDC 50419-407-01
1 Unit
Rx only
This product (like all oral contraceptives) is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
BEYAZ
(drospirenone/ethinyl estradiol/ levomefolate calcium tablets and levomefolate calcium tablets)www.beyaz.com
– 28 tablets
– oral
Close -
INGREDIENTS AND APPEARANCEProduct Information
BEYAZ drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50419-407 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50419-407-00 90 in 1 PACKAGE 10/07/2010 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:50419-407-03 3 in 1 PACKAGE 10/07/2010 2 NDC:50419-407-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:50419-407-75 12 in 1 CARTON 10/07/2010 3 5 in 1 PACKAGE 3 NDC:50419-407-71 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:50419-407-70 90 in 1 PACKAGE 10/07/2010 4 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 24 Part 2 4 Part 1 of 2 BEYAZ drospirenone, ethinyl estradiol and levomefolate calcium tablet, film coated Product Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DROSPIRENONE (UNII: N295J34A25) (DROSPIRENONE - UNII:N295J34A25) DROSPIRENONE 3 mg ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 0.02 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 0.451 mg Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BETADEX (UNII: JV039JZZ3A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color PINK Score no score Shape ROUND Size 6mm Flavor Imprint Code Z Contains 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022532 10/07/2010 Part 2 of 2 BEYAZ levomefolate calcium tablet, film coated Product Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 0.451 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color ORANGE (Light) Score no score Shape ROUND Size 6mm Flavor Imprint Code M Contains 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022532 10/07/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022532 10/07/2010 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Bayer Weimar GmbH und Co. KG 331485631 MANUFACTURE(50419-407) , ANALYSIS(50419-407) Establishment Name Address ID/FEI Business Operations Bayer AG 315097875 PACK(50419-407) , LABEL(50419-407) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 LABEL(50419-407) Establishment Name Address ID/FEI Business Operations Bayer AG 314398484 API MANUFACTURE(50419-407) , ANALYSIS(50419-407)
CloseEstablishment Name Address ID/FEI Business Operations Bayer AG 342872971 PARTICLE SIZE REDUCTION(50419-407) , ANALYSIS(50419-407)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
BEYAZ- drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit
Number of versions: 17
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Nov 13, 2024 | 18 (current) | download |
| Jun 26, 2023 | 17 | download |
| Nov 10, 2022 | 16 | download |
| May 12, 2022 | 15 | download |
| Sep 10, 2021 | 14 | download |
| Oct 7, 2020 | 13 | download |
| Feb 20, 2019 | 12 | download |
| Jan 3, 2019 | 11 | download |
| Sep 22, 2017 | 10 | download |
| Jun 15, 2015 | 9 | download |
| Jun 28, 2012 | 8 | download |
| May 16, 2012 | 7 | download |
| Feb 24, 2012 | 6 | download |
| Mar 2, 2011 | 5 | download |
| Feb 23, 2011 | 4 | download |
| Feb 22, 2011 | 3 | download |
| Oct 8, 2010 | 2 | download |
RxNorm
BEYAZ- drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1013626 | drospirenone 3 MG / ethinyl estradiol 0.02 MG / levomefolate calcium 0.451 MG Oral Tablet | PSN |
| 2 | 1013626 | drospirenone 3 MG / ethinyl estradiol 0.02 MG / levomefolate calcium 0.451 MG Oral Tablet | SCD |
| 3 | 1013628 | levomefolate calcium 0.451 MG Oral Tablet | PSN |
| 4 | 1013628 | levomefolate calcium 0.451 MG Oral Tablet | SCD |
| 5 | 1013629 | Drospiren-Eth estra-Levomefol Ca 3-0.02-0.451 MG (24) Oral Tablet / Levomefol Ca 0.451 MG (4) Oral Tablet 28 Day Pack | PSN |
| 6 | 1013629 | {24 (drospirenone 3 MG / ethinyl estradiol 0.02 MG / levomefolate calcium 0.451 MG Oral Tablet) / 4 (levomefolate calcium 0.451 MG Oral Tablet) } Pack | GPCK |
| 7 | 1013630 | Beyaz 28 Day Pack | PSN |
| 8 | 1013630 | {24 (drospirenone 3 MG / ethinyl estradiol 0.02 MG / levomefolate calcium 0.451 MG Oral Tablet) / 4 (levomefolate calcium 0.451 MG Oral Tablet) } Pack [Beyaz 28 Day] | BPCK |
| 9 | 1013630 | Beyaz 28 Day Pack | SY |
Get Label RSS Feed for this Drug
BEYAZ- drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=8132454a-6135-4bac-b206-83a55eb8dbc6
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
BEYAZ- drospirenone/ethinyl estradiol/levomefolate calcium and levomefolate calcium kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 50419-407-00 |
| 2 | 50419-407-01 |
| 3 | 50419-407-03 |
| 4 | 50419-407-70 |
| 5 | 50419-407-71 |
| 6 | 50419-407-75 |