Label: BELRAPZO- bendamustine hydrochloride injection
- NDC Code(s): 42367-521-25
- Packager: Eagle Pharmaceuticals, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BELRAPZO safely and effectively. See full prescribing information for BELRAPZO. BELRAPZO® (bendamustine hydrochloride ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Chronic Lymphocytic Leukemia (CLL) BELRAPZO is indicated for the treatment of patients with chronic lymphocytic leukemia. Efficacy relative to first line therapies other than chlorambucil ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Instructions for CLL - Recommended Dosage: The recommended dosage is 100 mg/m2 administered intravenously over 30 minutes on Days 1 and 2 of a 28-day cycle, up to 6 ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 100 mg/4 mL (25 mg/mL) as a clear and colorless to yellow ready-to dilute solution in a multiple-dose vial.
-
4 CONTRAINDICATIONSBELRAPZO is contraindicated in patients with a known hypersensitivity (e.g., anaphylactic and anaphylactoid reactions) to bendamustine, polyethylene glycol 400, propylene glycol, or ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - Bendamustine hydrochloride caused severe myelosuppression (Grade 3-4) in 98% of patients in the two NHL studies [see Adverse Reactions (6.1)]. Three patients (2%) died ...
-
6 ADVERSE REACTIONSThe following clinically significant serious adverse reactions are discussed in greater detail in other sections of the prescribing information. Myelosuppression [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on BELRAPZO - CYP1A2 Inhibitors - The coadministration of BELRAPZO with CYP1A2 inhibitors may increase bendamustine plasma concentrations and may result in increased ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - In animal reproduction studies, intraperitoneal administration of bendamustine to pregnant mice and rats during organogenesis at doses 0.6 to 1.8 times the maximum ...
-
10 OVERDOSAGEThe intravenous LD50 of bendamustine hydrochloride is 240 mg/m2 in the mouse and rat. Toxicities included sedation, tremor, ataxia, convulsions and respiratory distress. Across all clinical ...
-
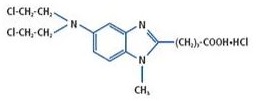
11 DESCRIPTIONBELRAPZO (bendamustine hydrochloride) is an alkylating agent. The chemical name of bendamustine hydrochloride is 1H-benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]-1-methyl- ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bendamustine is a bifunctional mechlorethamine derivative containing a purine-like benzimidazole ring. Mechlorethamine and its derivatives form electrophilic alkyl ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Bendamustine was carcinogenic in mice. After intraperitoneal injections at 37.5 mg/m2/day (the lowest dose tested, approximately 0.3 ...
-
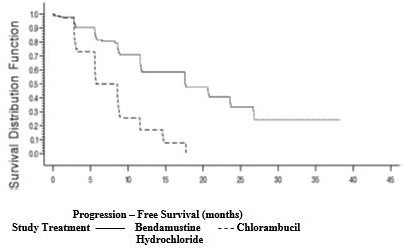
14 CLINICAL STUDIES14.1 Chronic Lymphocytic Leukemia (CLL) The safety and efficacy of bendamustine hydrochloride were evaluated in an open-label, randomized, controlled multicenter trial comparing bendamustine ...
-
15 REFERENCESOSHA Hazardous Drugs. OSHA. [http://www.osha.gov/SLTC/hazardousdrugs/index.html]
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSafe Handling and Disposal - BELRAPZO (bendamustine hydrochloride) is a hazardous drug. Follow applicable special handling and disposal procedures1. Care should be exercised in the handling and ...
-
17 PATIENT COUNSELING INFORMATIONMyelosuppression - Inform patients of the likelihood that BELRAPZO will cause a decrease in white blood cells, platelets, and red blood cells and the need for frequent monitoring of blood counts ...
-
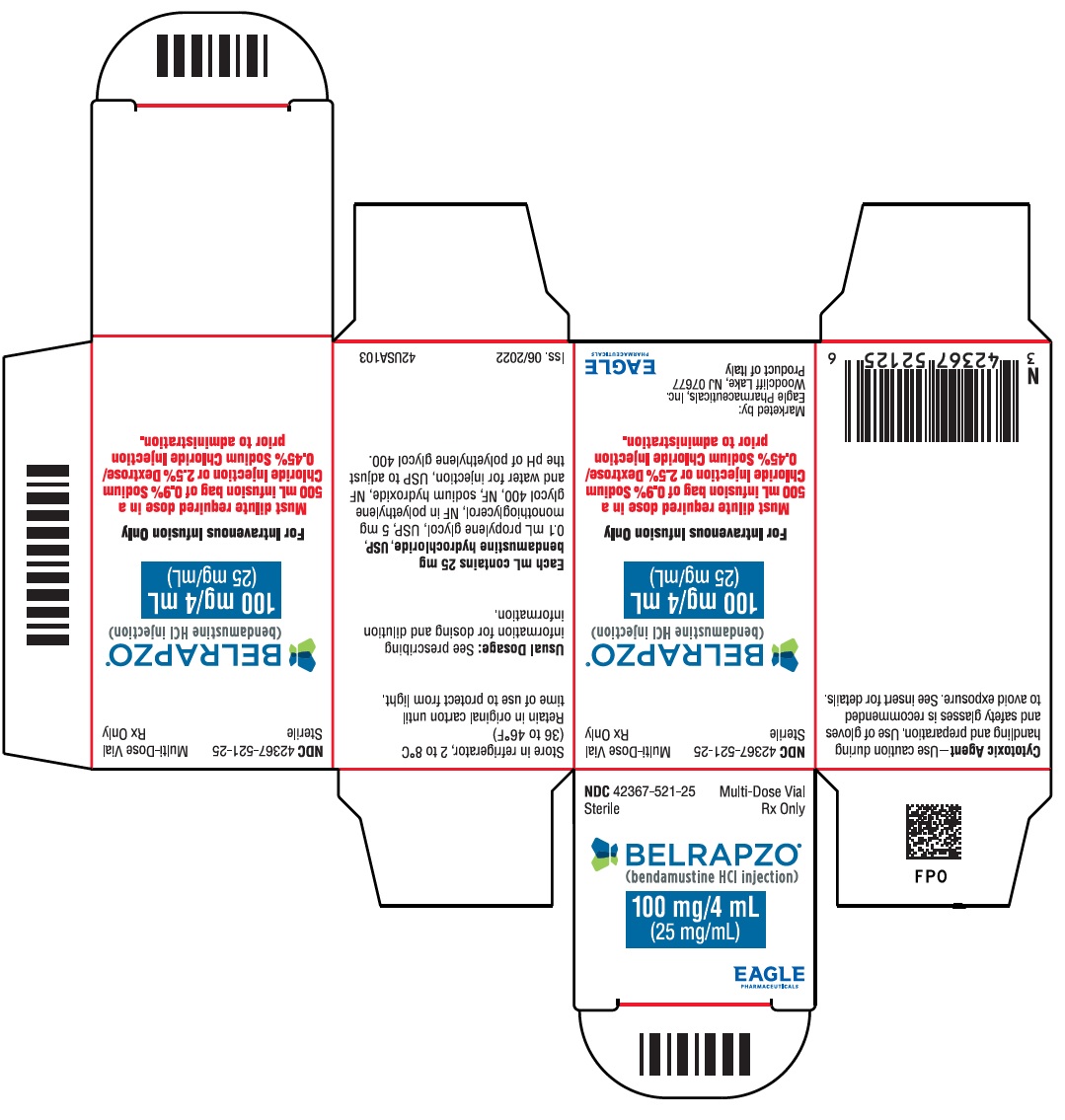
PRINCIPAL DISPLAY PANEL - NDC: 42367-521-25 - BSP Carton Label
-
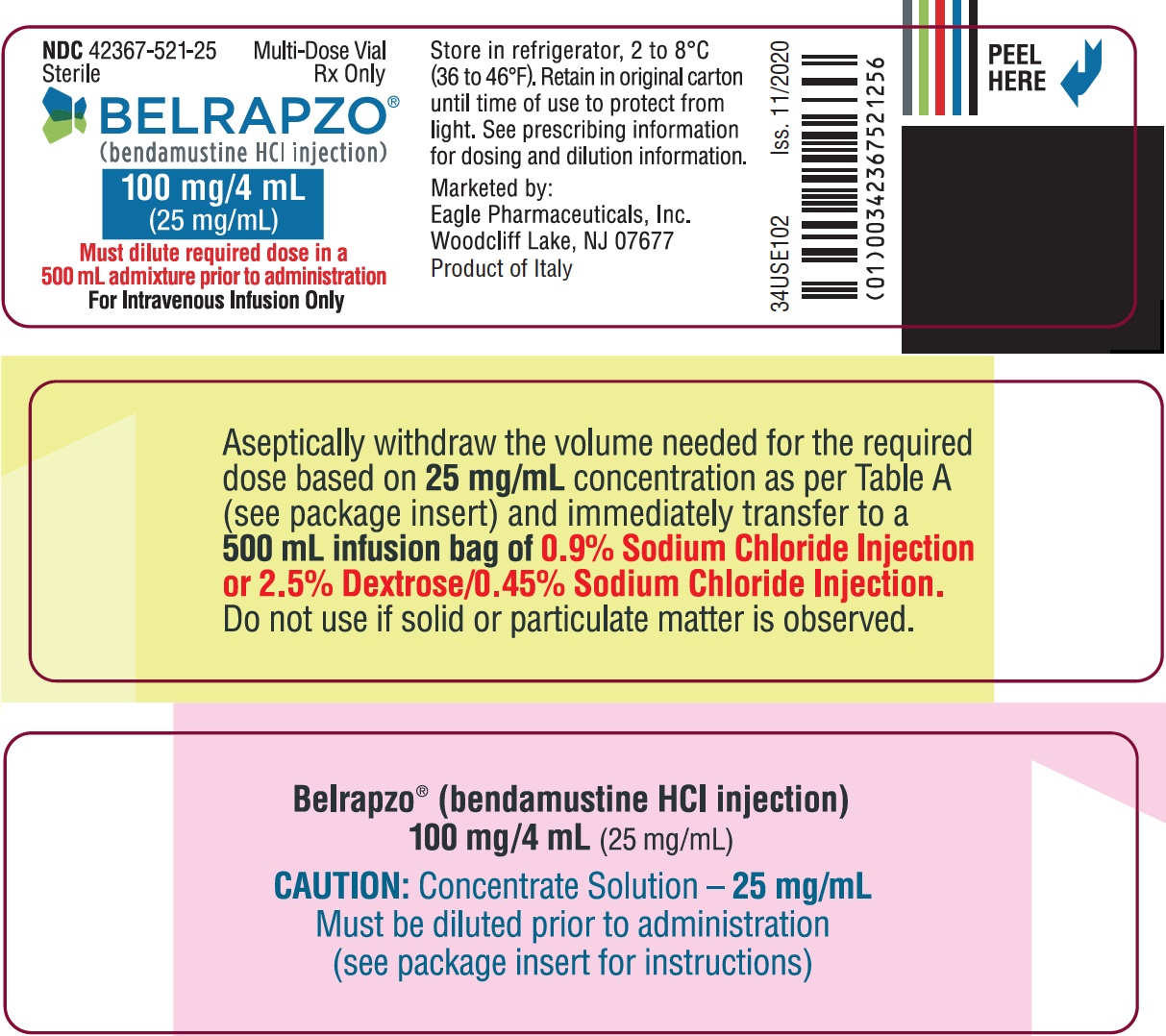
PRINCIPAL DISPLAY PANEL - NDC: 42367-521-25 - BSP Vial Label
-
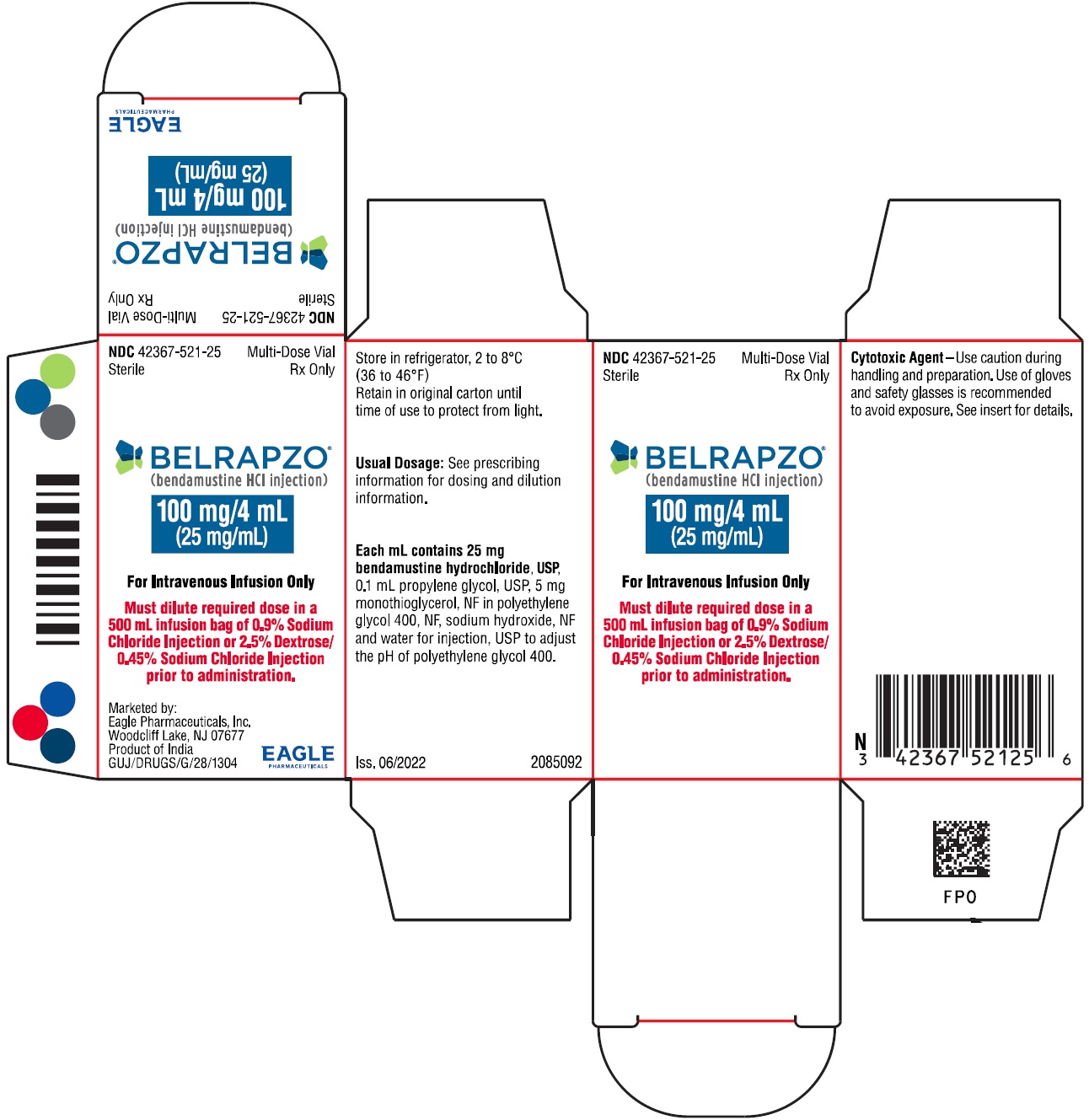
PRINCIPAL DISPLAY PANEL - NDC: 42367-521-25 - Zydus Carton Label
-
PRINCIPAL DISPLAY PANEL - NDC: 42367-521-25 - Cadila Vial Label

-
INGREDIENTS AND APPEARANCEProduct Information