Label: BELEODAQ- belinostat injection, powder, lyophilized, for solution
- NDC Code(s): 72893-002-01
- Packager: Acrotech Biopharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BELEODAQ safely and effectively. See full prescribing information for BELEODAQ. BELEODAQ® (belinostat) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBeleodaq is indicated for the treatment of adult patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). This indication is approved under accelerated approval based on tumor ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of Beleodaq is 1,000 mg/m2 administered over 30 minutes by intravenous infusion once daily on Days 1 through 5 of a 21-day cycle. Cycles can be ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 500 mg, lyophilized powder in single-dose vial for reconstitution
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Hematologic Toxicity - Beleodaq can cause thrombocytopenia, leukopenia (neutropenia and lymphopenia), and/or anemia; monitor blood counts weekly during treatment, and modify dosage as ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described in more detail in other sections of the prescribing information. • Hematologic Toxicity [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 UGT1A1 Inhibitors - Avoid concomitant administration of Beleodaq with UGT1A1inhibitors. If concomitant use of a UGT1A1 inhibitor is unavoidable, modify the Beleodaq dose [see Dosage and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, Beleodaq can cause teratogenicity and/or embryo-fetal lethality because it is genotoxic and targets actively dividing cells [see ...
-
10 OVERDOSAGENo specific information is available on the treatment of overdosage of Beleodaq. There is no antidote for Beleodaq and it is not known if Beleodaq is dialyzable. If an overdose occurs, general ...
-
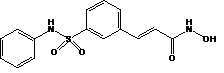
11 DESCRIPTIONBeleodaq is a histone deacetylase inhibitor with a sulfonamide-hydroxamide structure. The chemical name of belinostat is (2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide. The structural ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Beleodaq is a histone deacetylase (HDAC) inhibitor. HDACs catalyze the removal of acetyl groups from the lysine residues of histones and some non-histone proteins. In ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been performed with belinostat. Belinostat was genotoxic in a bacterial reverse mutation test (AMES ...
-
14 CLINICAL STUDIESRelapsed or Refractory Peripheral T-cell Lymphoma (PTCL) In an open-label, single-arm, non-randomized international trial conducted at 62 centers, 129 patients with relapsed or refractory PTCL ...
-
15 REFERENCES1 OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
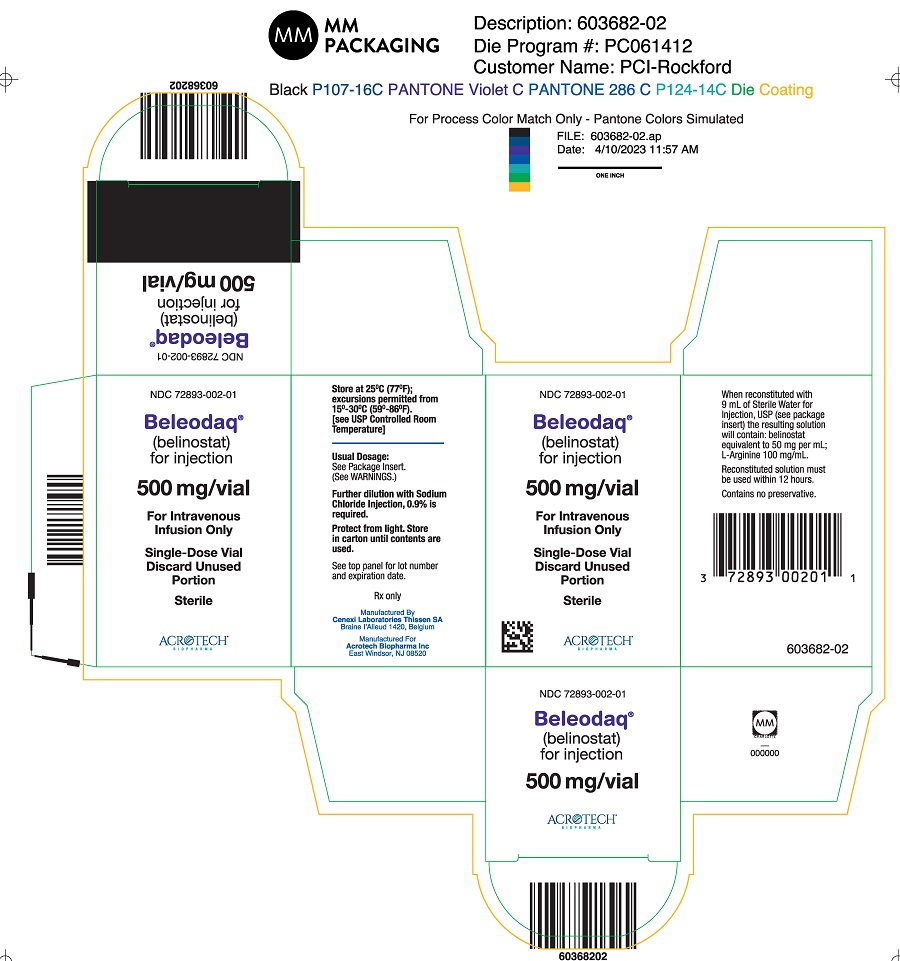
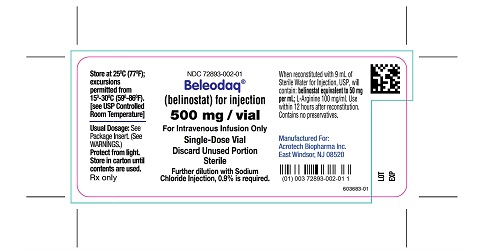
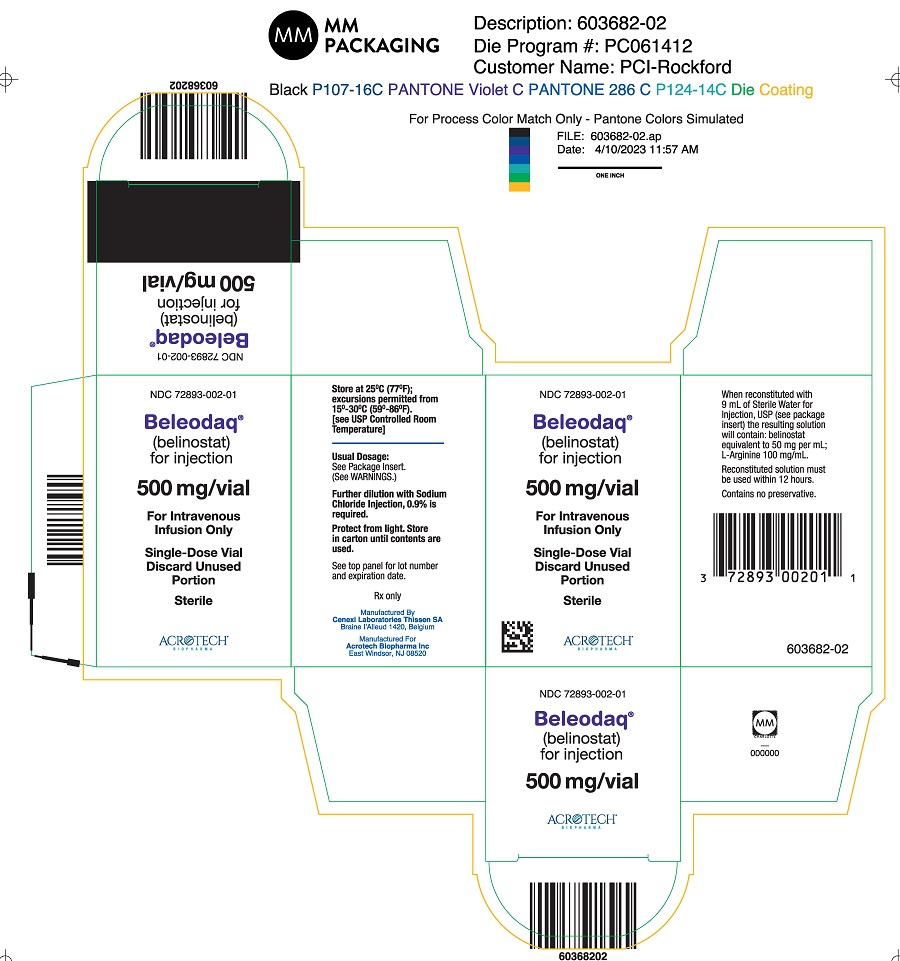
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Beleodaq (belinostat) for injection is supplied in single vial cartons; each clear vial contains sterile, lyophilized powder equivalent to 500 mg belinostat. NDC 72893-002-01 ...
-
17 PATIENT COUNSELING INFORMATIONPhysicians should discuss the FDA approved Patient Information Leaflet with patients prior to treatment with Beleodaq. Instruct patients to read the Patient Information Leaflet ...
-
SPL PATIENT PACKAGE INSERT SECTIONPATIENT INFORMATION - BELEODAQ®(Bē-lēo-dak) (belinostat) for injection, for intravenous use - Read this Patient Information before you receive treatment with Beleodaq and ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELCarton Label - NDC 72893-002-01 - Beleodaq® (belinostat) for injection - 500 mg/vial - For Intravenous Infusion Only - Single-Dose Vial - Vial Label - NDC 72893-002-01 - Beleodaq® (belinostat) for ...
-
INGREDIENTS AND APPEARANCEProduct Information