Label: BASIC DENTAL EMERGENCY KIT- epinephrine, albuterol sulfate, nitroglycerin, diphenhydramine hydrochloride, aspirin kit
- NDC Code(s): 83220-002-08
- Packager: Best Dental Kit LLC
- This is a repackaged label.
- Source NDC Code(s): 0280-2000
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONBasic Dental Emergency Kit - These highlights do not include all the information needed to use EPINEPHRINE INJECTION safely and effectively. See full prescribing information for EPINEPHRINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEEpinephrine injection is indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which includes bees, wasps ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage According to Patient Body Weight - Patients greater than or equal to 30 kg (approximately 66 pounds or more): 0.3 mg - Patients 15 kg to 30 kg (33 pounds to 66 pounds): 0.15 ...
-
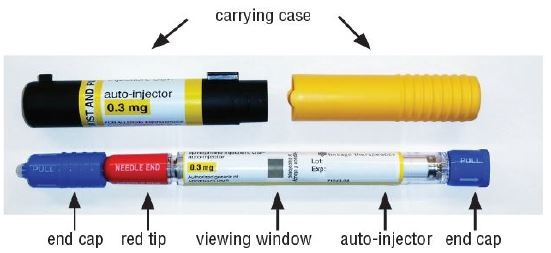
3 DOSAGE FORMS AND STRENGTHSInjection: 0.3 mg (0.3 mg/0.3 mL) of clear and colorless solution in single-dose pre-filled auto-injector
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Emergency Treatment - Epinephrine injection is intended for immediate administration as emergency supportive therapy and is not intended as a substitute for immediate medical care. In ...
-
6 ADVERSE REACTIONSDue to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine ...
-
7 DRUG INTERACTIONSCardiac Glycosides, Diuretics, and Anti-arrhythmics - Patients who receive epinephrine while concomitantly taking cardiac glycosides, diuretics, or anti-arrhythmics should be observed carefully ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available human data on the use of epinephrine injection in pregnant women to inform a drug-associated risk of adverse developmental outcomes. In ...
-
10 OVERDOSAGEOverdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in ...
-
11 DESCRIPTIONEpinephrine injection, USP 0.3 mg is an auto-injector and a combination product containing drug and device components. Each epinephrine injection, USP 0.3 mg delivers a single dose of 0.3 mg ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Epinephrine acts on both alpha- and beta-adrenergic receptors. 12.2 Pharmacodynamics - Through its action on alpha-adrenergic receptors, epinephrine lessens the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted. Epinephrine and other catecholamines ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGStorage and Handling - Protect from light. Epinephrine is light sensitive and should be stored in the carrying-case provided to protect it from light. Store at room temperature (20°C to 25°C (68°F ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (Patient Information and Instructions for Use) A healthcare provider should review the patient instructions and operation of epinephrine injection, in detail ...
-
Patient InformationEPINEPHRINE injection (ep-in-eph-rine), for intramuscular or subcutaneous use - For allergic emergencies (anaphylaxis) Read this Patient Information leaflet carefully before you use epinephrine ...
-
Instructions for UseEPINEPHRINE injection (ep-in-eph-rine) for intramuscular or subcutaneous use - For allergic emergencies (anaphylaxis) Read this Instructions for Use carefully before you use epinephrine injection ...
-
SPL UNCLASSIFIED SECTIONAlbuterol Sulfate Inhalation Aerosol HFA with Dose Indicator - FOR ORAL INHALATION ONLY - Prescribing Information
-
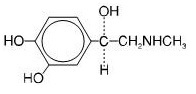
DESCRIPTION
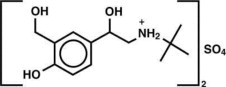
The active component of Albuterol Sulfate Inhalation Aerosol is albuterol sulfate, USP racemic α 1 [( tert-Butylamino)methyl]-4-hydroxy- m-xylene-α,α'-diol sulfate (2:1)(salt), a relatively ...
-
CLINICAL PHARMACOLOGY
Mechanism of Action - In vitro studies and - in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta ...
-
INDICATIONS AND USAGE
Albuterol Sulfate Inhalation Aerosol is indicated in adults and children 4 years of age and older for the treatment or prevention of bronchospasm with reversible obstructive airway disease and for ...
-
CONTRAINDICATIONS
Albuterol Sulfate Inhalation Aerosol is contraindicated in patients with a history of hypersensitivity to albuterol or any other Albuterol Sulfate Inhalation Aerosol components.
-
WARNINGS
Paradoxical Bronchospasm: Inhaled albuterol sulfate can produce paradoxical bronchospasm that may be life threatening. If paradoxical bronchospasm occurs, Albuterol Sulfate Inhalation Aerosol ...
-
PRECAUTIONS
General Albuterol sulfate, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias ...
-
ADVERSE REACTIONS
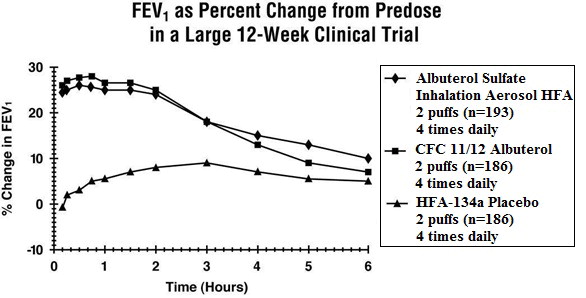
Adverse reaction information concerning Albuterol Sulfate Inhalation Aerosol is derived from a 12-week, double-blind, double-dummy study which compared Albuterol Sulfate Inhalation Aerosol, a CFC ...
-
OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under - ADVERSE ...
-
DOSAGE AND ADMINISTRATION
For treatment of acute episodes of bronchospasm or prevention of asthmatic symptoms, the usual dosage for adults and children 4 years of age and older is two inhalations repeated every 4 to ...
-
HOW SUPPLIED
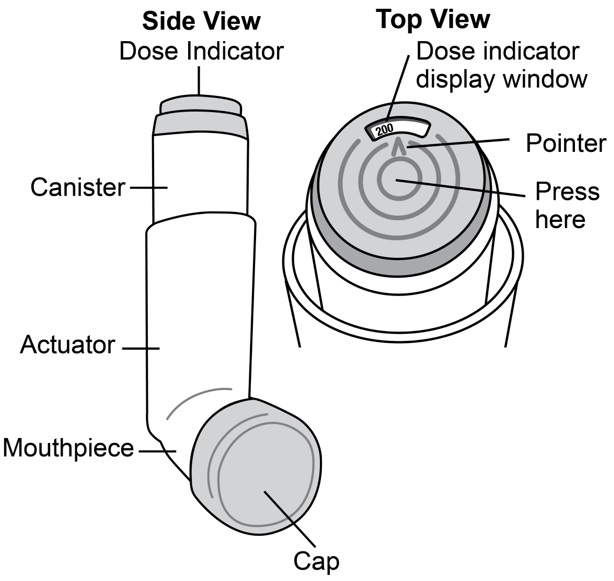
Albuterol Sulfate Inhalation Aerosol is supplied as a pressurized aluminum canister, with an attached dose indicator, a yellow plastic actuator and orange dust cap each in boxes of one. Each ...
-
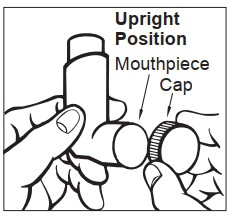
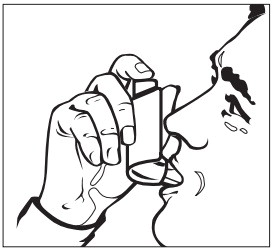
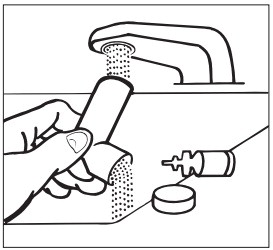
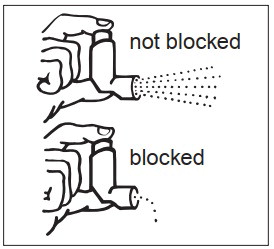
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Albuterol Sulfate (al-BYOO-ter-ole) Inhalation Aerosol with Dose Indicator - Read this Instructions for Use before you start using Albuterol Sulfate Inhalation Aerosol and ...
-
SPL UNCLASSIFIED SECTIONNITROGLYCERIN TABLETS - These highlights do not include all the information needed to use NITROGLYCERIN SUBLINGUAL TABLETS safely and effectively. See full prescribing information for ...
-
1 INDICATIONS AND USAGENitroglycerin sublingual tablets are indicated for the acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
-
2 DOSAGE AND ADMINISTRATIONAdminister one tablet under the tongue or in the buccal pouch at the first sign of an acute anginal attack. Allow tablet to dissolve without swallowing. One additional tablet may be administered ...
-
3 DOSAGE FORMS AND STRENGTHSNitroglycerin Sublingual Tablets, USP are supplied as white to off-white, round, flat-faced tablets in three strengths: 0.3 mg (Debossed with “1” on one side and “C” on the other) 0.4 mg (Debossed ...
-
4 CONTRAINDICATIONS4.1 PDE-5-Inhibitors and sGC-Stimulators - Do not use nitroglycerin sublingual tablets in patients who are taking PDE-5 Inhibitors, such as avanafil, sildenafil, tadalafil, vardenafil ...
-
5 WARNINGS AND PRECAUTIONSTolerance: Excessive use may lead to tolerance. (5.1) Hypotension: Severe hypotension may occur. (5.2) 5.1 Tolerance - Excessive use may lead to the development of tolerance. Only the smallest ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail elsewhere in the label: Hypotension [see Warnings and Precautions (5.2)] Headache [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSErgotamine: increased bioavailability of ergotamine. Avoid concomitant use. (7.2) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 10/2021 - 7.1 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal ...
-
10 OVERDOSAGE10.1 Signs and Symptoms, Methemoglobinemia - Nitrate overdosage may result in: severe hypotension, persistent throbbing headache, vertigo, palpitation, visual disturbance, flushing and perspiring ...
-
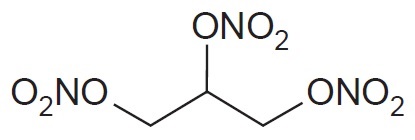
11 DESCRIPTIONNitroglycerin Sublingual Tablets, USP are stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg, or 0.6 mg nitroglycerin; as well as calcium stearate, croscarmellose ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3'5' monophosphate (cyclic GMP) in smooth ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed. Carcinogenicity potential of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNitroglycerin Sublingual Tablets, USP are supplied as white to off white, round, flat-faced tablets in 3 strengths (0.3 mg, 0.4 mg, and 0.6 mg) in bottles. Store at 20°C to 25°C (68°F to 77°F ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). This product's label may have been updated. For full prescribing information, please visit ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Glenmark Pharmaceuticals Limited - India - Manufactured for: Glenmark Pharmaceuticals Inc., USA - Mahwah, NJ 07430 - Questions? 1 (888) 721-7115 - www.glenmarkpharma-us.com - October ...
-
Patient InformationNitroglycerin (nahy-truh-glis-er-in) Sublingual Tablets, USP - Read this information carefully before you start nitroglycerin sublingual tablets and each time you refill your prescription. There ...
-
SPL UNCLASSIFIED SECTIONDIPHENHYDRAMINE HYDROCHLORIDE INJECTION, USP - Rx only
-
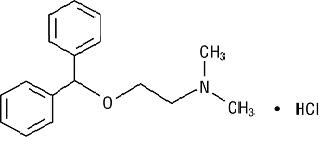
DESCRIPTIONDiphenhydramine Hydrochloride Injection is a sterile, nonpyrogenic solution for intravenous or deep intramuscular use as an antihistaminic agent. Each mL contains diphenhydramine hydrochloride 50 ...
-
CLINICAL PHARMACOLOGYDiphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector ...
-
INDICATIONS AND USAGEDiphenhydramine Hydrochloride Injection is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when the oral form is ...
-
CONTRAINDICATIONSUse in Neonates or Premature Infants - This drug should not be used in neonates or premature infants. Use in Nursing Mothers - Because of the higher risk of antihistamines for infants ...
-
WARNINGSAntihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy or ...
-
PRECAUTIONSGeneral - Diphenhydramine hydrochloride has an atropine-like action and, therefore, should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure ...
-
ADVERSE REACTIONSThe most frequent adverse reactions are italicized. General - Urticaria; drug rash; anaphylactic shock; photosensitivity; excessive perspiration; chills; dryness of mouth, nose and ...
-
OVERDOSAGEAntihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in pediatric patients. Atropine-like signs and symptoms, dry ...
-
DOSAGE AND ADMINISTRATIONTHIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY. Diphenhydramine Hydrochloride Injection is indicated when the oral form is impractical. DOSAGE SHOULD BE INDIVIDUALIZED ...
-
HOW SUPPLIEDDiphenhydramine Hydrochloride Injection, USP 50 mg/mL - Storage - Protect from light. Keep covered in carton until time of use. Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to ...
-
SPL UNCLASSIFIED SECTIONBAYER GENUINE ASPIRIN TABLETS - Drug Facts
-
Active ingredient (in each tablet)
Aspirin 325 mg (NSAID)1 - 1nonsteroidal anti-inflammatory drug
-
Purposes
Pain reliever/fever reducer
-
Uses
temporarily relieves minor aches and pains due to: headache - muscle pain - toothache - menstrual pain - colds - minor pain of arthritis - temporarily reduces fever
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea ...
-
Directions
drink a full glass of water with each dose - adults and children 12 years and over: take 1 or 2 tablets every 4 hours or 3 tablets every 6 hours, not to exceed 12 tablets in 24 hours - children under ...
-
Other information
save carton for full directions and warnings - store at room temperature
-
Inactive ingredients
corn starch, hypromellose, powdered cellulose, triacetin
-
Questions?
1-800-331-4536 (Mon-Fri 9AM-5PM EST)
-
SPL UNCLASSIFIED SECTIONThis product is repackaged by: Select Corporation - 1433 Wainwright Way, Carrollton, TX 75007 - 877-244-4400 M - F 9am - 5 pm, from a product manufactured by Bayer. Dist. by: Bayer HealthCare ...
-
TRUEplus GLUCOSE TABLETS15 g Fast acting Carbohydrates per Serving - Raises low blood sugar and boosts energy - Fat Free - Gluten Free - Sodium Free - ORANGE NATURALLY & ARTIFICIALLY FLAVORED - DO NOT USE IF PROTECTIVE SEAL ...
-
DIRECTIONSChew desired amount of glucose. Store at room temperature. Do not refrigerate or freeze. Do not expose to excessive heat or moisture.
-
SPL UNCLASSIFIED SECTIONDistributed by McKesson - 6555, State Highway 161, Las Colinas, TX 75039 - TRUEplus is a trademark of Trivida Health, Inc. healthmart.com
-
SPL UNCLASSIFIED SECTIONKIT CONTAINS: EPINEPHRINE AUTO-INJECT (0.3 mg) – 1 no. ALBUTEROL SULFATE HFA INHALER (90 mcg) – 1 no. NITROGLYCERIN SUBLINGUAL TABLETS (0.4 mg) – 1 bottle (25 tablets) DIPHENHYDRAMINE HCl ...
-
Packaging
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information