Label: BABYBIG- human botulinum neurotoxin a/b immune globulin injection, powder, lyophilized, for solution
- NDC Code(s): 68403-1100-6, 68403-1100-7
- Packager: CALIFORNIA DEPARTMENT OF PUBLIC HEALTH
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BabyBIG, [Botulism Immune Globulin Intravenous (Human)], safely and effectively. See full prescribing information for BabyBIG ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBabyBIG®, Botulism Immune Globulin Intravenous (Human), is indicated for the treatment of infant botulism caused by toxin type A or B in patients below one year of age.
-
2 DOSAGE AND ADMINISTRATIONFor Intravenous Use Only - 2.1 Preparation for Administration - BabyBIG does not contain a preservative. After reconstitution of the lyophilized product, the vial should be entered only once for ...
-
3 DOSAGE FORMS AND STRENGTHS100 mg ± 20 mg lyophilized immunoglobulin per single-dose vial
-
4 CONTRAINDICATIONSAs with other immunoglobulin preparations, BabyBIG should not be used in individuals with a prior history of severe reaction to other human immunoglobulin preparations.[1-4] Individuals with ...
-
5 WARNINGS AND PRECAUTIONSOnly administer BabyBIGas an intravenous infusion, since other routes of administration have not been evaluated. Do not use BabyBIG if the reconstituted solution is turbid [see DOSAGE AND ...
-
6 ADVERSE REACTIONSSerious adverse reactions were not observed in clinical trials using BabyBIG. The most common adverse reaction observed with BabyBIG treatment during clinical trials (>5%) was skin rash. Other ...
-
7 DRUG INTERACTIONSAdmixtures of BabyBIG with other drugs have not been evaluated. It is recommended that BabyBIG be administered separately from other drugs or medications that the patient may be receiving [see ...
-
8 USE IN SPECIFIC POPULATIONS8.4 Pediatric Use - BabyBIG has been studied for safety and efficacy only in patients below one year of age [see ADVERSE REACTIONS (6) and CLINICAL STUDIES (14)]. It has not been tested in other ...
-
10 OVERDOSAGEAlthough limited data are available, clinical experience with other immunoglobulin preparations suggests that the major manifestations would be those related to volume overload.[1]
-
11 DESCRIPTIONBabyBIG, Botulism Immune Globulin Intravenous (Human) (BIG-IV), is a solvent-detergent-treated, sterile, lyophilized powder of immunoglobulin G (IgG), stabilized with 5% sucrose and 1% albumin ...
-
12 CLINICAL PHARMACOLOGYBabyBIG contains IgG antibodies from the immunized donors who contributed to the plasma pool from which the product was derived. The titer of antibodies in the reconstituted product against type A ...
-
14 CLINICAL STUDIESTwo clinical studies in infant botulism were performed:[16] (1) an adequate and well-controlled study to evaluate the safety and efficacy of BabyBIG (N=129), and (2) an open label study to collect ...
-
15 REFERENCESCytogam®, cytomegalovirus immune globulin intravenous (human) (CMV-IGIV). 55th edition. Physician's Desk Reference. 2001, Montvale, New Jersey: Medical Economics Company, Inc. 1861-1863. Immune ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNDC 68403-1100-6, 100 mg ± 20 mg lyophilized immunoglobulin single-dose vial individually packaged in a carton. Store the vial containing the lyophilized product between 2° and 8°C (35.6° to ...
-
17 PATIENT COUNSELING INFORMATIONDiscuss the risks and benefits of BabyBIG use with the patient's legal guardians, including the possibility of adverse reactions, e.g., hypersensitivity reactions such as anaphylaxis, as well as ...
-
SPL UNCLASSIFIED SECTIONFor additional information concerning BabyBIG, contact: Infant Botulism Treatment and Prevention Program - California Department of Public Health - 850 Marina Bay Parkway, Room E-361 - Richmond ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 2 mL Vial Label - Botulism Immune Globulin Intravenous - (Human) (BIG-IV) BabyBIG® DO NOT SHAKE VIAL - AFTER RECONSTITUTION; AVOID FOAMING. See package insert ...
-
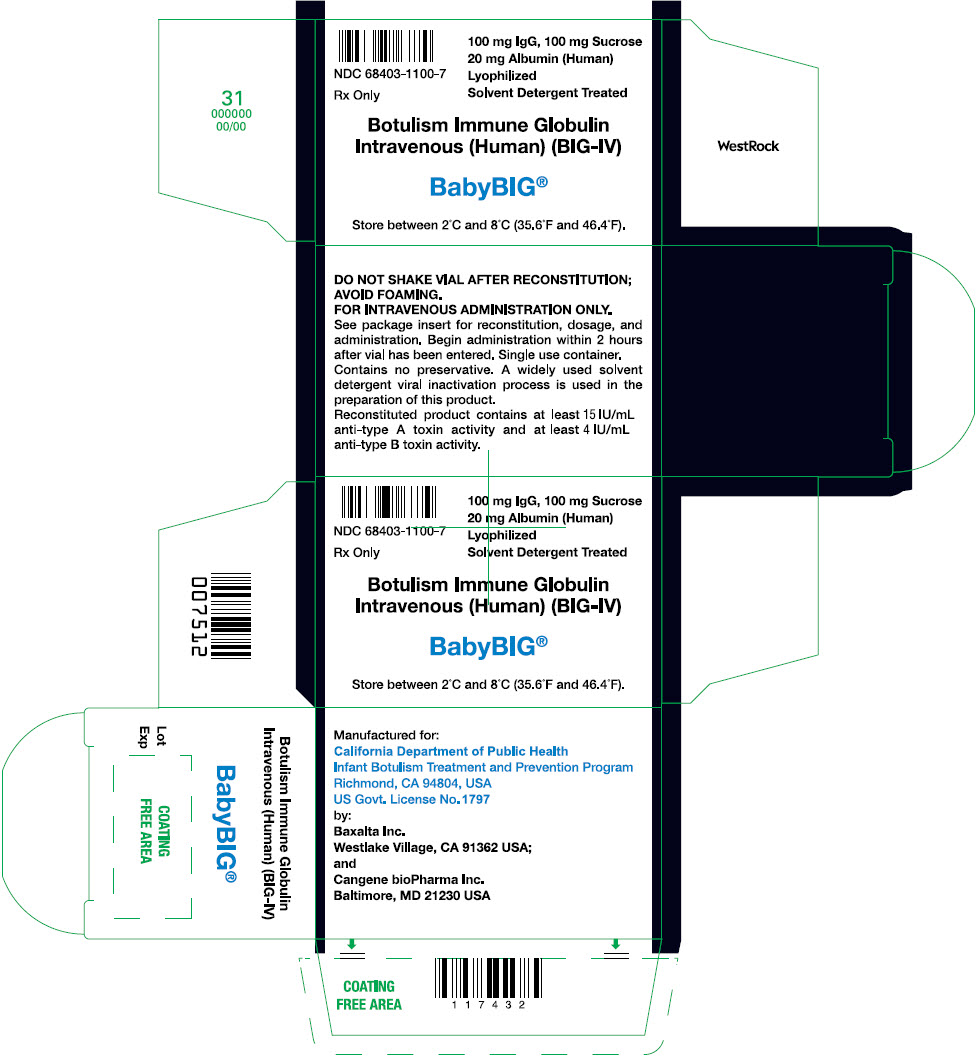
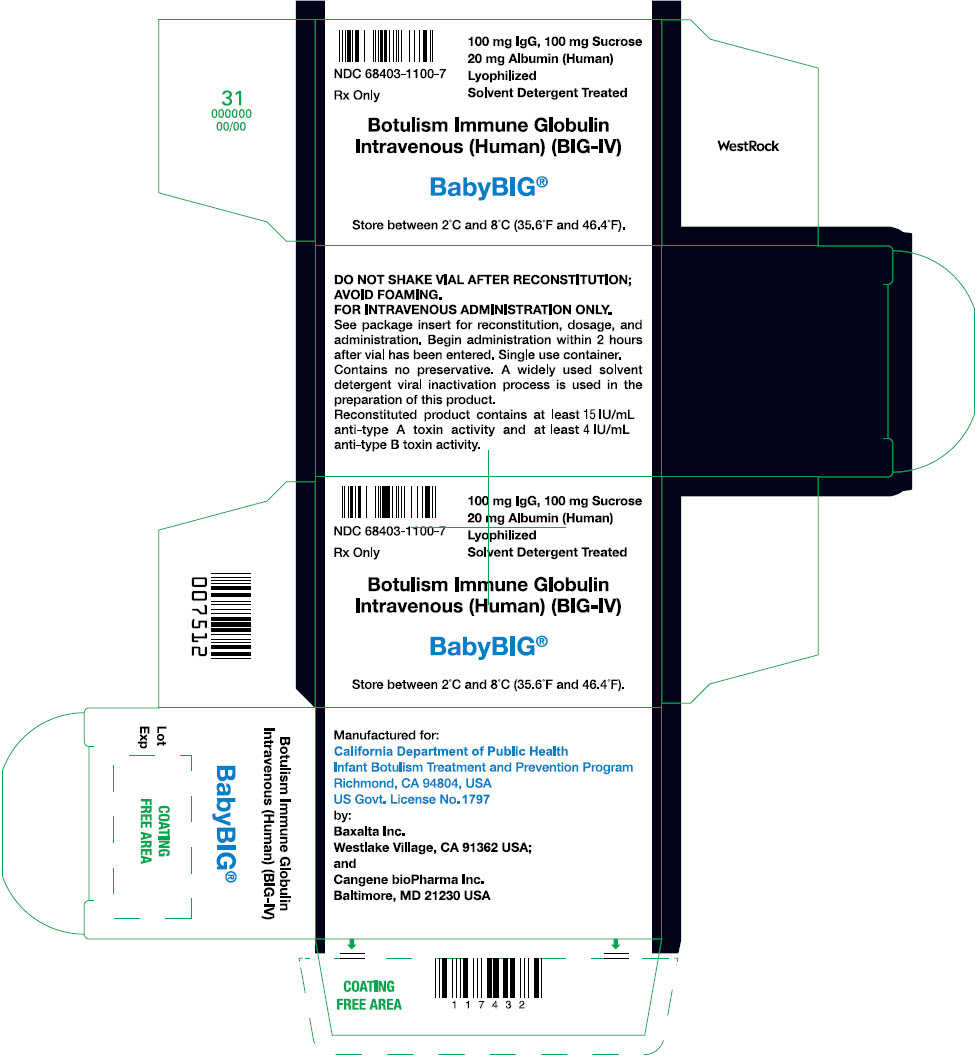
PRINCIPAL DISPLAY PANEL - 1 Single Use Vial CartonNDC 68403-1100-7 - Rx Only - 100 mg IgG, 100 mg Sucrose - 20 mg Albumin (Human) Lyophilized - Solvent Detergent Treated - Botulism Immune Globulin - Intravenous (Human) (BIG-IV) BabyBIG® Store between 2°C ...
-
INGREDIENTS AND APPEARANCEProduct Information