Label: AZACTAM- aztreonam injection, powder, for solution
- NDC Code(s): 0003-2560-16, 0003-2570-16
- Packager: E.R. Squibb & Sons, L.L.C.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of AZACTAM® and other antibacterial drugs, AZACTAM should be used only to treat or prevent infections that are ...
-
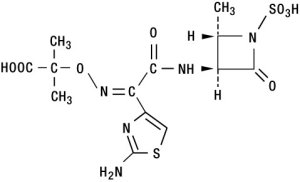
DESCRIPTIONAZACTAM® (aztreonam for injection, USP) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal ...
-
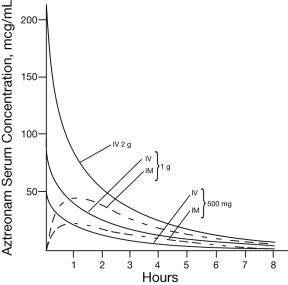
CLINICAL PHARMACOLOGYSingle 30-minute intravenous infusions of 500 mg, 1 g, and 2 g doses of AZACTAM in healthy subjects produced aztreonam peak serum levels of 54 mcg/mL, 90 mcg/mL, and 204 mcg/mL, respectively ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of AZACTAM (aztreonam for injection, USP) and other antibacterial drugs, AZACTAM should be used only to treat or ...
-
CONTRAINDICATIONSThis preparation is contraindicated in patients with known hypersensitivity to aztreonam or any other component in the formulation.
-
WARNINGSBoth animal and human data suggest that AZACTAM (aztreonam for injection, USP) is rarely cross-reactive with other beta-lactam antibiotics and weakly immunogenic. Treatment with aztreonam can ...
-
PRECAUTIONSGeneral - Prescribing AZACTAM in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the ...
-
ADVERSE REACTIONSLocal reactions such as phlebitis/thrombophlebitis following intravenous administration, and discomfort/swelling at the injection site following intramuscular administration occurred at rates of ...
-
OVERDOSAGEIf necessary, aztreonam may be cleared from the serum by hemodialysis and/or peritoneal dialysis.
-
DOSAGE AND ADMINISTRATIONDosage in Adult Patients - AZACTAM may be administered intravenously or by intramuscular injection. Dosage and route of administration should be determined by susceptibility of the causative ...
-
HOW SUPPLIEDAZACTAM® (aztreonam for injection, USP) Single-dose vials: • 1 gram/vial: Packages of 10 - NDC 0003-2560-16 - • 2 grams/vial: Packages of 10 - NDC 0003-2570-16 - Storage - Store in ...
-
SPL UNCLASSIFIED SECTIONAZACTAM and the Bristol-Myers Squibb logo are registered trademarks of Bristol-Myers Squibb Company. All other trademarks are the property of their respective owners. Manufactured ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL REPRESENTATIVE PACKAGING - See How Supplied section for a complete list of available packages of AZACTAM. NDC 0003-2560-16 - 1 gram - AZACTAM® (aztreonam for injection, USP) FOR ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0003-2570-16 - 2 grams - AZACTAM® (aztreonam for injection, USP) FOR INTRAVENOUS - OR - INTRAMUSCULAR USE - Single-Dose vial - Discard Unused Portion - Rx only - Bristol-Myers Squib
-
INGREDIENTS AND APPEARANCEProduct Information