Label: AXUMIN- fluciclovine f-18 injection, solution
- NDC Code(s): 69932-001-30, 69932-001-50
- Packager: Blue Earth Diagnostics
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine F 18) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAxumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior ...
-
2 DOSAGE AND ADMINISTRATION2.1 Radiation Safety - Drug Handling - Axumin is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration [see - Warnings ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: supplied as a clear, colorless solution in a 30 mL or 50 mL multiple-dose vial containing 335 MBq/mL to 8,200 MBq/mL (9 mCi/mL to 221 mCi/mL) fluciclovine F 18 at calibration time and ...
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Risk for Image Misinterpretation - Image interpretation errors can occur with Axumin PET imaging. A negative image does not rule out the presence of recurrent prostate cancer and a positive ...
-
6 ADVERSE REACTIONSClinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Axumin is not indicated for use in females and there is no information on the risk of adverse development outcomes in pregnant women or animals with the use of ...
-
10 OVERDOSAGEIn case of overdose of Axumin, encourage patients to maintain hydration and to void frequently to minimize radiation exposure.
-
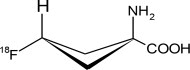
11 DESCRIPTION11.1 Chemical Characteristics - Axumin contains the fluorine 18 (F 18) labeled synthetic amino acid analog fluciclovine. Fluciclovine F 18 is a radioactive diagnostic agent used with PET imaging ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of action - Fluciclovine F 18 is a synthetic amino acid transported across mammalian cell membranes by amino acid transporters, such as LAT-1 and ASCT2, which are upregulated in ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - No long term studies in animals have been performed to evaluate the carcinogenic potential of ...
-
14 CLINICAL STUDIESThe safety and efficacy of Axumin were evaluated in two studies (Study 1 and Study 2) in men with suspected recurrence of prostate cancer based on rising PSA levels following radical prostatectomy ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Axumin is supplied as a clear, colorless injection in a 30 mL or 50 mL multiple-dose glass vial containing approximately 26 mL solution of 335 MBq/mL to 8,200 MBq/mL (9 mCi/mL ...
-
17 PATIENT COUNSELING INFORMATIONInstruct patients to avoid significant exercise for at least a day before the PET scan. Instruct patients not to eat or drink for at least 4 hours before the PET scan (other than sips of water ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 30 mL Multiple-Dose Vial Label - Sterile - Axumin™ Non-pyrogenic - 335 MBq/mL to 8,200 MBq/mL (9 mCi/mL to - 221 mCi/mL) at End of Synthesis (EOS) Diagnostic ...
-
INGREDIENTS AND APPEARANCEProduct Information