Label: AVEED- testosterone undecanoate injection
- NDC Code(s): 67979-511-43
- Packager: ENDO USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: New Drug Application
Drug Label Information
Updated August 28, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AVEED® safely and effectively. See full prescribing information for AVEED®. AVEED® (testosterone undecanoate) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS PULMONARY OIL MICROEMBOLISM (POME) REACTIONS AND ANAPHYLAXIS
- Serious POME reactions, involving urge to cough, dyspnea, throat tightening, chest pain, dizziness, and syncope; and episodes of anaphylaxis, including life-threatening reactions, have been reported to occur during or immediately after the administration of testosterone undecanoate injection. These reactions can occur after any injection of testosterone undecanoate during the course of therapy, including after the first dose [see Warnings and Precautions (5.1)].
- Following each injection of AVEED, observe patients in the healthcare setting for 30 minutes in order to provide appropriate medical treatment in the event of serious POME reactions or anaphylaxis [see Warnings and Precautions (5.1)].
- Because of the risks of serious POME reactions and anaphylaxis, AVEED is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the AVEED REMS Program [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
AVEED is indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone. Primary hypogonadism (congenital or ...
-
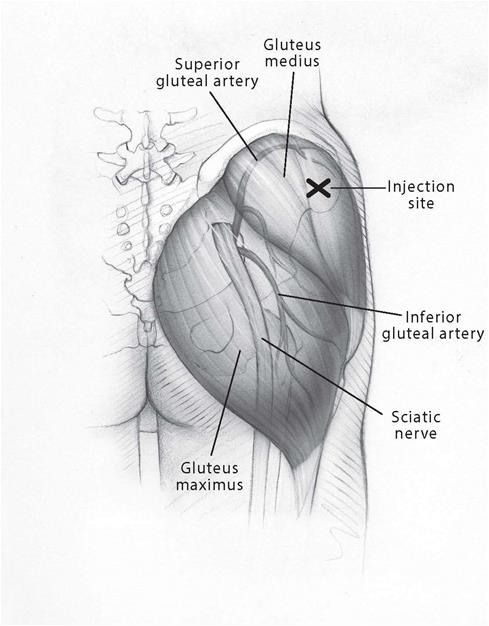
2 DOSAGE AND ADMINISTRATION
Prior to initiating AVEED, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least 2 separate days and that these ...
-
3 DOSAGE FORMS AND STRENGTHS
750 mg/3 mL (250 mg/mL) testosterone undecanoate sterile injectable solution is provided in an amber glass, single use vial with silver-colored crimp seal and gray plastic cap.
-
4 CONTRAINDICATIONS
AVEED should not be used in any of the following patients: Men with carcinoma of the breast or known or suspected carcinoma of the prostate [see Warnings and Precautions (5.3)]. Women who are ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Pulmonary Oil Microembolism (POME) Reactions and Anaphylaxis - Serious POME reactions, involving cough, urge to cough, dyspnea, hyperhidrosis, throat tightening, chest pain ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 Insulin - Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - AVEED is contraindicated in pregnant women. Testosterone is teratogenic and may cause fetal harm based on data from animal studies and its mechanism of action ...
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance - AVEED contains testosterone, a Schedule III controlled substance in the Controlled Substances Act. 9.2 Abuse - Drug abuse is intentional non-therapeutic use of ...
-
10 OVERDOSAGE
There have been no reports of overdosage in the AVEED clinical trials. There is 1 report of acute overdosage with use of an approved injectable testosterone product: this subject had serum ...
-
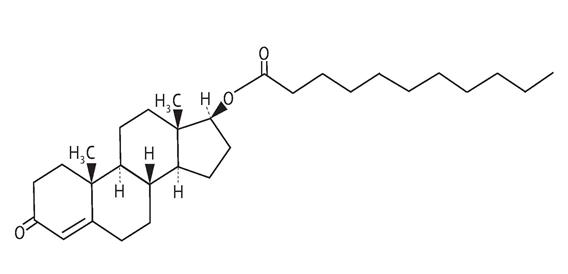
11 DESCRIPTION
AVEED (testosterone undecanoate) injection contains testosterone undecanoate (17β-undecanoyloxy-4-androsten-3-one) which is an ester of the androgen, testosterone. Testosterone is formed by ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Endogenous androgens, including testosterone and dihydrotestosterone (DHT) are responsible for the normal growth and development of the male sex organs and for ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant ...
-
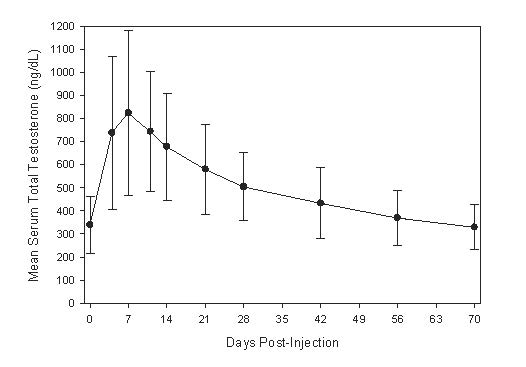
14 CLINICAL STUDIES
14.1 Testosterone Replacement Therapy - AVEED was evaluated for efficacy in an 84-week, single-arm, open-label, multicenter study of 130 hypogonadal men. Eligible patients weighed at least 65 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
AVEED, NDC 67979-511-43: 750 mg/3 mL (250 mg/mL) testosterone undecanoate sterile injectable solution is provided in an amber glass vial with silver-colored crimp seal and gray plastic cap. Each ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Medication Guide. Advise patients of the following: 17.1 Risks of Serious Pulmonary Oil Microembolism (POME) and Anaphylaxis - Serious POME reactions, involving cough ...
-
MEDICATION GUIDEMEDICATION GUIDE - AVEED® (Uh-Veed) (testosterone undecanoate) injection - Read this Medication Guide before you receive AVEED and before each injection. There may be new information. This ...
-
PRINCIPAL DISPLAY PANELPrinciple Display Panel - Vial Label
-
PRINCIPAL DISPLAY PANELPrinciple Display Panel - Carton
-
INGREDIENTS AND APPEARANCEProduct Information