Label: AYVAKIT- avapritinib tablet, film coated
-
NDC Code(s):
72064-110-30,
72064-120-30,
72064-125-30,
72064-130-30, view more72064-150-30
- Packager: Blueprint Medicines Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AYVAKIT safely and effectively. See full prescribing information for AYVAKIT. AYVAKIT® (avapritinib) tablets, for oral use ...These highlights do not include all the information needed to use AYVAKIT safely and effectively. See full prescribing information for AYVAKIT.
AYVAKIT® (avapritinib) tablets, for oral use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
AYVAKIT is a kinase inhibitor indicated for:
Gastrointestinal Stromal Tumor (GIST)
- the treatment of adults with unresectable or metastatic GIST harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation, including PDGFRA D842V mutations. (1.1, 2.2)
Advanced Systemic Mastocytosis (AdvSM)
- the treatment of adult patients with AdvSM. AdvSM includes patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematological neoplasm (SM-AHN), and mast cell leukemia (MCL). (1.2)
- Limitations of Use: AYVAKIT is not recommended for the treatment of patients with AdvSM with platelet counts of less than 50 × 109/L (1.2)
Indolent Systemic Mastocytosis (ISM)
DOSAGE AND ADMINISTRATION
- GIST: Select patients for treatment with AYVAKIT based on the presence of a PDGFRA exon 18 mutation. (2.2)
- GIST: The recommended dosage is 300 mg orally once daily. (2.2)
- AdvSM: The recommended dosage is 200 mg orally once daily. (2.3)
- ISM: The recommended dosage is 25 mg orally once daily. (2.4)
- Patients with severe hepatic impairment (Child-Pugh Class C): reduce dose of AYVAKIT. (2.7)
DOSAGE FORMS AND STRENGTHS
Tablets: 25 mg, 50 mg, 100 mg, 200 mg and 300 mg. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Intracranial Hemorrhage: Permanently discontinue for any occurrence of any grade. (2.5, 5.1)
- Cognitive Effects: A broad spectrum of cognitive adverse reactions can occur in patients receiving AYVAKIT. In patients with GIST, AdvSM, or ISM depending on the severity, continue AYVAKIT at same dose, withhold and then resume at same or reduced dose upon improvement, or permanently discontinue. (2.5, 5.2)
- Photosensitivity: May cause photosensitivity reactions. Advise patients to limit direct ultraviolet exposure. (5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females and males of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.4, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions are:
- GIST (≥20% incidence): edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, increased lacrimation, abdominal pain, constipation, rash, dizziness, and hair color changes. (6.1)
- AdvSM (≥20% incidence): edema, diarrhea, nausea, and fatigue/asthenia. (6.1)
- ISM (≥10% incidence): eye edema, dizziness, peripheral edema and flushing. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Blueprint Medicines Corporation at 1-888-258-7768 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong and Moderate CYP3A Inhibitors: Avoid coadministration of AYVAKIT with strong and moderate CYP3A inhibitors. If coadministration of AYVAKIT with a moderate inhibitor cannot be avoided, reduce dose of AYVAKIT in patients with GIST or AdvSM. (2.6, 7.1)
- Strong and Moderate CYP3A Inducers: Avoid coadministration of AYVAKIT with strong and moderate CYP3A inducers. (7.1)
- Hormonal contraceptives containing ethinyl estradiol: See full prescribing information for dose-specific recommendations for concomitant use (7.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 PDGFRA Exon 18 Mutation-Positive Unresectable or Metastatic Gastrointestinal Stromal Tumor (GIST)
1.2 Advanced Systemic Mastocytosis (AdvSM)
1.3 Indolent Systemic Mastocytosis (ISM)
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Administration
2.2 GIST Harboring PDGFRA Exon 18 Mutations
2.3 Advanced Systemic Mastocytosis
2.4 Indolent Systemic Mastocytosis
2.5 Dosage Modifications for Adverse Reactions
2.6 Concomitant Use of Strong and Moderate CYP3A Inhibitors
2.7 Dosage Modifications for Severe Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Intracranial Hemorrhage
5.2 Cognitive Effects
5. 3 Photosensitivity
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on AYVAKIT
7.2 Effects of AYVAKIT on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Gastrointestinal Stromal Tumors
14.2 Advanced Systemic Mastocytosis
14.3 Indolent Systemic Mastocytosis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 PDGFRA Exon 18 Mutation-Positive Unresectable or Metastatic Gastrointestinal Stromal Tumor (GIST) AYVAKIT® is indicated for the treatment of adults with unresectable or metastatic GIST ...
1.1 PDGFRA Exon 18 Mutation-Positive Unresectable or Metastatic Gastrointestinal Stromal Tumor (GIST)
AYVAKIT® is indicated for the treatment of adults with unresectable or metastatic GIST harboring a platelet-derived growth factor receptor alpha (PDGFRA) exon 18 mutation, including PDGFRA D842V mutations [see Dosage and Administration (2.2)].
1.2 Advanced Systemic Mastocytosis (AdvSM)
AYVAKIT is indicated for the treatment of adult patients with advanced systemic mastocytosis (AdvSM). AdvSM includes patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematological neoplasm (SM-AHN), and mast cell leukemia (MCL).
Limitations of Use:
AYVAKIT is not recommended for the treatment of patients with AdvSM with platelet counts of less than 50 × 109/L [see Warnings and Precautions (5.1)].
Close1.3 Indolent Systemic Mastocytosis (ISM)
AYVAKIT is indicated for the treatment of adult patients with indolent systemic mastocytosis (ISM).
Limitations of Use:
AYVAKIT is not recommended for the treatment of patients with ISM with platelet counts of less than 50 × 109/L [see Warnings and Precautions (5.1)].
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Administration - Administer AYVAKIT orally on an empty stomach, at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology (12.3)]. Do not make up for a missed ...
2.1 Recommended Administration
Administer AYVAKIT orally on an empty stomach, at least 1 hour before or 2 hours after a meal [see Clinical Pharmacology (12.3)].
Do not make up for a missed dose within 8 hours of the next scheduled dose.
Do not repeat dose if vomiting occurs after AYVAKIT but continue with the next scheduled dose.
2.2 GIST Harboring PDGFRA Exon 18 Mutations
Select patients for treatment with AYVAKIT based on the presence of a PDGFRA exon 18 mutation [see Clinical Studies (14.1)]. An FDA-approved test for the detection of exon 18 mutations is not currently available.
The recommended dosage of AYVAKIT is 300 mg orally once daily in patients with GIST. Continue treatment until disease progression or unacceptable toxicity.
2.3 Advanced Systemic Mastocytosis
The recommended dosage of AYVAKIT is 200 mg orally once daily in patients with AdvSM. Continue treatment until disease progression or unacceptable toxicity.
2.4 Indolent Systemic Mastocytosis
The recommended dosage of AYVAKIT is 25 mg orally once daily in patients with ISM.
2.5 Dosage Modifications for Adverse Reactions
The recommended dosage reductions and modifications for adverse reactions are provided in Tables 1 and 2.
Table 1: Recommended Dosage Reductions for AYVAKIT for Adverse Reactions Dose Reduction Level Dosage in patients with GIST* Dosage in patients with AdvSM† First dose reduction 200 mg once daily 100 mg once daily Second dose reduction 100 mg once daily 50 mg once daily Third dose reduction - 25 mg once daily Table 2: Recommended Dosage Modifications for AYVAKIT for Adverse Reactions Adverse Reaction Severity* Dosage Modification - *
- Severity as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0
Patients with GIST or AdvSM Intracranial Hemorrhage [see Warnings and Precautions (5.1)] Any grade Permanently discontinue AYVAKIT. Cognitive Effects [see Warnings and Precautions (5.2)] Grade 1 Continue AYVAKIT at same dose or reduced dose or withhold until improvement to baseline or resolution. Resume at same dose or reduced dose. Grade 2 or Grade 3 Withhold AYVAKIT until improvement to baseline, Grade 1, or resolution. Resume at same dose or reduced dose. Grade 4 Permanently discontinue AYVAKIT. Other [see Adverse Reactions (6.1)] Grade 3 or Grade 4 Withhold AYVAKIT until improvement to less than or equal to Grade 2. Resume at same dose or reduced dose, as clinically appropriate. Patients with AdvSM Thrombocytopenia [see Warnings and Precautions (5.1)] <50 × 109/L Interrupt AYVAKIT until platelet count is ≥ 50 × 109/L, then resume at reduced dose (per Table 1). If platelet counts do not recover above 50 × 109/L, consider platelet support. 2.6 Concomitant Use of Strong and Moderate CYP3A Inhibitors
Avoid concomitant use of AYVAKIT with strong or moderate CYP3A inhibitors. If concomitant use with a moderate CYP3A inhibitor cannot be avoided, the starting dosage of AYVAKIT is as follows [see Drug Interactions (7.1)]:
- GIST: 100 mg orally once daily
- AdvSM: 50 mg orally once daily
For ISM, avoid concomitant use of AYVAKIT with strong or moderate CYP3A inhibitors.
Close2.7 Dosage Modifications for Severe Hepatic Impairment
A modified starting dosage of AYVAKIT is recommended for patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7)]:
- GIST: 200 mg orally once daily
- AdvSM: 100 mg orally once daily
- ISM: 25 mg orally every other day
-
3 DOSAGE FORMS AND STRENGTHSTablets: 25 mg, round, white film-coated tablet with debossed text. One side reads "BLU" and the other side reads "25". 50 mg, round, white film-coated tablet with debossed text. One side reads ...
Tablets:
- 25 mg, round, white film-coated tablet with debossed text. One side reads "BLU" and the other side reads "25".
- 50 mg, round, white film-coated tablet with debossed text. One side reads "BLU" and the other side reads "50".
- 100 mg, round, white film-coated, printed with blue ink "BLU" on one side and "100" on the other side.
- 200 mg, capsule shaped, white film-coated, printed with blue ink "BLU" on one side and "200" on the other side.
- 300 mg, capsule shaped, white film-coated, printed with blue ink "BLU" on one side and "300" on the other side.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Intracranial Hemorrhage - Serious intracranial hemorrhage may occur with AYVAKIT treatment; fatal events occurred in less than 1% of patients. Overall, intracranial hemorrhage (e.g. ...
5.1 Intracranial Hemorrhage
Serious intracranial hemorrhage may occur with AYVAKIT treatment; fatal events occurred in less than 1% of patients. Overall, intracranial hemorrhage (e.g., subdural hematoma, intracranial hemorrhage, and cerebral hemorrhage) occurred in 2.9% of the 749 patients with GIST or AdvSM who received AYVAKIT in clinical trials. No events of intracranial hemorrhage occurred in the 246 patients with ISM who received any dose of AYVAKIT in the PIONEER study.
Monitor patients closely for risk factors of intracranial hemorrhage which may include history of vascular aneurysm, intracranial hemorrhage or cerebrovascular accident within the prior year, concomitant use of anticoagulant drugs, or thrombocytopenia.
Symptoms of intracranial hemorrhage may include headache, nausea, vomiting, vision changes, or altered mental status. Advise patients to seek immediate medical attention for signs or symptoms of intracranial hemorrhage.
Permanently discontinue AYVAKIT if intracranial hemorrhage of any grade occurs [see Dosage and Administration (2.5)].
Gastrointestinal Stromal Tumors
Intracranial hemorrhage occurred in 3 of 267 patients (1.1%). Two (0.7%) of the events were Grade ≥ 3 and resulted in discontinuation of study drug. Events of intracranial hemorrhage occurred in a range from 1.7 months to 19.3 months after initiating AYVAKIT.
Advanced Systemic Mastocytosis
In patients with AdvSM who received AYVAKIT at 200 mg daily, intracranial hemorrhage occurred in 2 of 75 patients (2.7%) who had platelet counts ≥ 50 × 109/L prior to initiation of therapy and in 3 of 80 patients (3.8%) regardless of platelet counts.
In patients with AdvSM, a platelet count must be performed prior to initiating therapy; AYVAKIT is not recommended in patients with AdvSM with platelet counts < 50 × 109/L. Following treatment initiation, platelet counts must be performed every 2 weeks for the first 8 weeks regardless of baseline platelet count. After 8 weeks of treatment, monitor platelet counts every 2 weeks (or more frequently as clinically indicated) if values are less than 75 × 109/L, every 4 weeks if values are between 75 and 100 × 109/L, and as clinically indicated if values are greater than 100 × 109/L.
Manage platelet counts of < 50 × 109/L by treatment interruption or dose-reduction of AYVAKIT. Platelet support may be necessary [see Dosage and Administration (2.5)]. Dose-interruptions and dose-reductions for thrombocytopenia occurred in 20% and 22% of AYVAKIT-treated patients, respectively. Thrombocytopenia was generally reversible by reducing or interrupting AYVAKIT.
5.2 Cognitive Effects
Cognitive adverse reactions can occur in patients receiving AYVAKIT. These cognitive adverse reactions occurred in 33% of the 995 patients with GIST, AdvSM or ISM who received AYVAKIT in clinical trials. These adverse reactions were managed with dose interruption and/or reduction when needed.
Overall, 10% led to dose interruptions, 7% led to dose reductions and 2.2% led to permanent discontinuation of AYVAKIT treatment in patients with GIST, AdvSM or ISM.
Depending on the severity and indication, withhold AYVAKIT and then resume at the same dose or at a reduced dose upon improvement, or permanently discontinue AYVAKIT [see Dosage and Administration (2.5)].
Indolent Systemic Mastocytosis
Cognitive adverse reactions occurred in 7.8% of patients with ISM who received AYVAKIT + best supportive care versus 7% of patients who received placebo + best supportive care in the PIONEER study; <1% were Grade 3. The median time to onset of the first cognitive adverse reaction was 2.3 months (range: 0 to 5.4 months). Median time to improvement to Grade 1 or complete resolution was 2.1 months (range: 0.4 to 2.1 months).
Gastrointestinal Stromal Tumors
Cognitive adverse reactions occurred in 41% of 601 patients with GIST who received AYVAKIT; 5% were Grade ≥ 3. Memory impairment occurred in 21% of patients; <1% of these events were Grade 3. Cognitive disorder occurred in 12% of patients; 1.2% of these events were Grade 3. Confusional state occurred in 6% of patients; <1% of these events were Grade 3. Amnesia occurred in 3% of patients; <1% of these events were Grade 3. Somnolence and speech disorder occurred in 2% of patients; none of these events were Grade 3. Other events occurred in less than 2% of patients.
The median time to onset of the first cognitive adverse reaction was 8.4 weeks (range: 1 day to 4 years). Among patients who experienced a cognitive effect of Grade 2 or worse (impacting activities of daily living), the median time to improvement to Grade 1 or complete resolution was 7.9 weeks. Overall, 2.7% of all patients who received AYVAKIT required permanent discontinuation for a cognitive adverse reaction, 13.5% required a dosage interruption, and 8.5% required dose reduction.
Advanced Systemic Mastocytosis
Cognitive adverse reactions occurred in 28% of 148 patients with AdvSM who received AYVAKIT; 3% were Grade ≥ 3. Memory impairment occurred in 16% of patients; all events were Grade 1 or 2. Cognitive disorder occurred in 10% of patients; <1% of these events were Grade 3. Confusional state occurred in 6% of patients; <1% of these events were Grade 3. Other events occurred in less than 2% of patients.
The median time to onset of the first cognitive adverse reaction was 13.3 weeks (range: 1 day to 1.8 years). Among patients who experienced a cognitive effect of Grade 2 or worse (impacting activities of daily living), the median time to improvement to Grade 1 or complete resolution was 8.1 weeks. Overall, 2% of all patients who received AYVAKIT required permanent discontinuation for a cognitive adverse reaction, 8.1% required a dosage interruption, and 8.8% required dose reduction.
5. 3 Photosensitivity
AYVAKIT may cause photosensitivity reactions. In all patients treated with AYVAKIT in clinical trials (n=1049), photosensitivity reactions occurred in 2.5% of patients. Advise patients to limit direct ultraviolet exposure during treatment with AYVAKIT and for one week after discontinuation of treatment.
Close5.4 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, AYVAKIT can cause fetal harm when administered to pregnant women. Oral administration of avapritinib during the period of organogenesis was teratogenic and embryotoxic in rats at exposures approximately 31.4, 6.3 and 2.7 times the human exposure based on area under the curve (AUC) at the 25 mg, 200 mg, and 300 mg dose, respectively.
Advise pregnant women of the potential risk to a fetus. Advise females and males of reproductive potential to use effective contraception during treatment with AYVAKIT and for 6 weeks after the final dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Intracranial hemorrhage [see Warnings and Precautions (5.1)] Cognitive effects [see Warnings and ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Intracranial hemorrhage [see Warnings and Precautions (5.1)]
- Cognitive effects [see Warnings and Precautions (5.2)]
- Photosensitivity [see Warnings and Precautions (5.3)]
Close6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS reflect exposure to AYVAKIT at 25 mg to 600 mg orally once daily in 995 patients enrolled in one of five clinicals trials conducted in patients with advanced malignancies and systemic mastocytosis, including NAVIGATOR, EXPLORER, PATHFINDER and PIONEER [see Clinical Studies (14.1, 14.2, 14.3)]. These patients included 601 patients with GIST, 148 patients with AdvSM and 246 patients with ISM. Among the 995 patients receiving AYVAKIT, 54% were exposed for 6 months or longer and 26% were exposed for greater than 1 year.
Gastrointestinal Stromal Tumors
Unresectable or Metastatic GIST
The safety of AYVAKIT in patients with unresectable or metastatic GIST was evaluated in NAVIGATOR [see Clinical Studies (14.1)]. The trial excluded patients with history of cerebrovascular accident or transient ischemic attacks, known risk of intracranial bleeding, and metastases to the brain. Patients received AYVAKIT 300 mg or 400 mg orally once daily (n = 204). Among patients receiving AYVAKIT, 56% were exposed for 6 months or longer and 44% were exposed for greater than one year.
The median age of patients who received AYVAKIT was 62 years (range: 29 to 90 years), 60% were <65 years, 62% were male, and 69% were White. Patients had received a median of 3 prior kinase inhibitors (range: 0 to 7).
Serious adverse reactions occurred in 52% of patients receiving AYVAKIT. Serious adverse reactions occurring in ≥1% of patients who received AYVAKIT were anemia (9%), abdominal pain (3%), pleural effusion (3%), sepsis (3%), gastrointestinal hemorrhage (2%), vomiting (2%), acute kidney injury (2%), pneumonia (1%), and tumor hemorrhage (1%). Fatal adverse reactions occurred in 3.4% of patients. Fatal adverse reactions that occurred in more than one patient were sepsis and tumor hemorrhage (1% each).
Permanent discontinuation due to adverse reactions occurred in 16% of patients who received AYVAKIT. Adverse reactions requiring permanent discontinuation in more than one patient were fatigue, abdominal pain, vomiting, sepsis, anemia, acute kidney injury, and encephalopathy.
Dosage interruptions due to an adverse reaction occurred in 57% of patients who received AYVAKIT. Adverse reactions requiring dosage interruption in >2% of patients who received AYVAKIT were anemia, fatigue, nausea, vomiting, hyperbilirubinemia, memory impairment, diarrhea, cognitive disorder, and abdominal pain.
Dose reduction due to an adverse reaction occurred in 49% of patients who received AYVAKIT. Median time to dose reduction was 9 weeks. Adverse reactions requiring dosage reduction in more than 2% of patients who received AYVAKIT were fatigue, anemia, hyperbilirubinemia, memory impairment, nausea, and periorbital edema.
The most common adverse reactions (≥ 20%) were edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, increased lacrimation, abdominal pain, constipation, rash, dizziness, and hair color changes. Table 5 summarizes the adverse reactions observed in NAVIGATOR.
Table 5. Adverse Reactions (≥ 10%) in Patients with GIST Receiving AYVAKIT in NAVIGATOR Adverse Reactions* AYVAKIT
N=204All Grades
%Grade ≥ 3
%- *
- Per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 and 5.0
- †
- Edema includes face swelling, conjunctival edema, eye edema, eyelid edema, orbital edema, periorbital edema, face edema, mouth edema, pharyngeal edema, peripheral edema, edema, generalized edema, localized edema, peripheral swelling, testicular edema.
- ‡
- Abdominal pain includes abdominal pain, upper abdominal pain, abdominal discomfort, lower abdominal pain, abdominal tenderness, and epigastric discomfort.
- §
- Cognitive impairment includes memory impairment, cognitive disorder, confusional state, disturbance in attention, amnesia, mental impairment, mental status changes, encephalopathy, dementia, abnormal thinking, mental disorder, and retrograde amnesia.
- ¶
- Sleep disorders includes insomnia, somnolence, and sleep disorder.
- #
- Taste effects include dysgeusia and ageusia.
- Þ
- Mood disorders includes agitation, anxiety, depression, depressed mood, dysphoria, irritability, mood altered, nervousness, personality change, and suicidal ideation.
- ß
- Rash includes rash, rash maculo-papular, rash erythematous, rash macular, rash generalized, and rash papular.
General Edema† 72 2 Fatigue/asthenia 61 9 Pyrexia 14 0.5 Gastrointestinal Nausea 64 2.5 Vomiting 38 2 Diarrhea 37 4.9 Abdominal pain‡ 31 6 Constipation 23 1.5 Dyspepsia 16 0 Nervous System Cognitive impairment§ 48 4.9 Dizziness 22 0.5 Headache 17 0.5 Sleep disorders¶ 16 0 Taste effects# 15 0 Mood disordersÞ 13 1 Metabolism and nutrition Decreased appetite 38 2.9 Eye Increased lacrimation 33 0 Skin and subcutaneous tissue Rashß 23 2.1 Hair color changes 21 0.5 Alopecia 13 0 Respiratory, thoracic and mediastinal Dyspnea 17 2.5 Pleural effusion 12 2 Investigations Weight decreased 13 1 Clinically relevant adverse reactions occurring in <10% of patients were:
Vascular: hypertension (8%)
Endocrine: thyroid disorders (hyperthyroid, hypothyroid) (3%)
Skin and subcutaneous: palmar-plantar erythrodysesthesia (1%)
Table 6 summarizes the laboratory abnormalities observed in NAVIGATOR.
Table 6. Select Laboratory Abnormalities (≥ 10%) Worsening from Baseline in Patients with GIST Receiving AYVAKIT in NAVIGATOR Laboratory Abnormality AYVAKIT*
N=204All Grades
(%)Grade ≥ 3
(%)- *
- The denominator used to calculate the rate varied from 154 to 201 based on the number of patients with a baseline value and at least one post-treatment value.
Hematology Decreased hemoglobin 81 28 Decreased leukocytes 62 5 Decreased neutrophils 43 6 Decreased platelets 27 0.5 Increased INR 24 0.6 Increased activated partial thromboplastin time 13 0 Chemistry Increased bilirubin 69 9 Increased aspartate aminotransferase 51 1.5 Decreased phosphate 49 13 Decreases potassium 34 6 Decreased albumin 31 2 Decreased magnesium 29 1 Increased creatinine 29 0 Decreased sodium 28 7 Increased alanine aminotransferase 19 0.5 Increased alkaline phosphatase 14 1 Advanced Systemic Mastocytosis
The safety of AYVAKIT in patients with AdvSM was evaluated in EXPLORER and PATHFINDER [see Clinical Studies (14.2)]. Patients received a starting dose of AYVAKIT ranging from 30 mg to 400 mg orally once daily (n = 131), including 80 patients who received the recommended starting dose of 200 mg once daily. Among patients receiving AYVAKIT, 70% were treated for 6 months or longer and 37% were exposed for greater than one year.
The median age of patients who received AYVAKIT was 68 years (range: 31 to 88 years), 38% were <65 years, 57% were male, and 88% were White.
Serious adverse reactions occurred in 34% of patients receiving the recommended starting dose of 200 mg once daily and in 50% of patients receiving AYVAKIT at all doses. Serious adverse reactions occurring in ≥1% of patients who received AYVAKIT were anemia (5%), subdural hematoma (4%), pleural effusion, ascites and pneumonia (3% each), acute kidney injury, gastrointestinal hemorrhage, intracranial hemorrhage, encephalopathy, gastric hemorrhage, large intestine perforation, pyrexia, and vomiting (2% each). Fatal adverse reactions occurred in 2.5% of patients receiving the recommended starting dose of 200 mg once daily and in 5.3% of patients receiving AYVAKIT at all doses. No specific adverse reaction leading to death was reported in more than one patient.
Permanent discontinuation due to adverse reactions occurred in 10% of patients receiving the recommended starting dose of 200 mg once daily and in 15% of patients who received AYVAKIT at all doses. Of patients receiving 200 mg once daily, subdural hematoma was the only adverse reaction requiring permanent discontinuation in more than one patient.
Dosage interruptions due to an adverse reaction occurred in 60% of patients receiving the recommended starting dose of 200 mg once daily and in 67% of patients who received AYVAKIT at all doses. Adverse reactions requiring dosage interruption in >2% of patients who received AYVAKIT at 200 mg once daily were thrombocytopenia, neutropenia, neutrophil count decreased, platelet count decreased, anemia, white blood cell decreased, cognitive disorder, blood alkaline phosphatase increased, and edema peripheral.
Dose reduction due to an adverse reaction occurred in 68% of patients receiving the recommended starting dose of 200 mg once daily and 70% of patients who received AYVAKIT at all doses. Median time to dose reduction was 1.7 months. Adverse reactions requiring dosage reduction in more than 2% of patients who received AYVAKIT at 200 mg once daily were thrombocytopenia, neutropenia, edema peripheral, neutrophil count decreased, platelet count decreased, periorbital edema, cognitive disorder, anemia, fatigue, arthralgia, blood alkaline phosphatase increased, and white blood cell count decreased.
The most common adverse reactions (≥ 20%) at all doses were edema, diarrhea, nausea, and fatigue/asthenia. Table 7 summarizes the adverse reactions observed in EXPLORER and PATHFINDER.
Table 7. Adverse Reactions (≥ 10%) in Patients with AdvSM Receiving AYVAKIT in EXPLORER and PATHFINDER Adverse Reactions* AYVAKIT (200 mg once daily)
N=80All Grades
%Grade ≥ 3
%- *
- Per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 and 5.0
- †
- Edema includes face swelling, eyelid edema, orbital edema, periorbital edema, face edema, peripheral edema, edema, generalized edema, and peripheral swelling.
- ‡
- Abdominal pain includes abdominal pain, upper abdominal pain, and abdominal discomfort.
- §
- Cognitive effects include memory impairment, cognitive disorder, confusional state, delirium, and disorientation.
- ¶
- Taste effects include dysgeusia.
General Edema† 79 5 Fatigue/asthenia 23 4 Gastrointestinal Diarrhea 28 1 Nausea 24 1 Vomiting 18 3 Abdominal pain‡ 14 1 Constipation 11 0 Nervous system Headache 15 0 Cognitive effects§ 14 1 Taste effects¶ 13 0 Dizziness 13 0 Musculoskeletal and connective tissue Arthralgia 10 1 Respiratory, thoracic and mediastinal Epistaxis 11 0 Clinically relevant adverse reactions occurring in <10% of patients were:
Cardiac: cardiac failure (2.5%), and cardiac failure congestive (1.3%)
Gastrointestinal: ascites (5%), gastrointestinal hemorrhage (1.3%), and large intestine perforation (1.3%)
Hepatobiliary: cholelithiasis (1.3%)
Infections and infestations: upper respiratory tract infection (6%), urinary tract infection (6%), and herpes zoster (2.5%)
Vascular: flushing (3.8%), hypertension (3.8%), hypotension (3.8%), and hot flush (2.5%)
Nervous: insomnia (6%)
Musculoskeletal and connective tissue: pain in extremity (6%)
Respiratory, thoracic and mediastinal: dyspnea (9%), and cough (2.5%)
Skin and subcutaneous tissue: rash1 (8%), alopecia (9%), pruritus (8%), and hair color changes (6%)
Metabolism and nutrition: decreased appetite (8%)
Eye: lacrimation increased (9%)
Laboratory abnormality: decreased phosphate (9%)
Rash includes rash and rash maculo-papular
Table 8 summarizes the laboratory abnormalities observed in EXPLORER and PATHFINDER.
Table 8. Select Laboratory Abnormalities (≥ 10%) Worsening from Baseline in Patients with AdvSM Receiving AYVAKIT in EXPLORER and PATHFINDER Laboratory Abnormality AYVAKIT (200 mg once daily)
N=80All Grades
(%)Grade ≥ 3
(%)Hematology Decreased platelets 64 21 Decreased hemoglobin 55 23 Decreased neutrophils 54 25 Decreased lymphocytes 34 11 Increased activated partial thromboplastin time 14 1 Increased lymphocytes 10 0 Chemistry Decreased calcium 50 3 Increased bilirubin 41 3 Increased aspartate aminotransferase 38 1 Decreased potassium 26 4 Increased alkaline phosphatase 24 5 Increased creatinine 20 0 Increased alanine aminotransferase 18 1 Decreased sodium 18 1 Decreased albumin 15 1 Decreased magnesium 14 1 Increased potassium 11 0
- 1
- Grouped terms
Other Clinically Relevant Adverse Reactions in <10% of patients
In the pooled GIST and AdvSM safety populations, photosensitivity occurred in 2.5% of patients [see Warnings and Precautions (5.3)].
Indolent Systemic Mastocytosis
The safety of AYVAKIT in patients with ISM was evaluated in PIONEER [see Clinical Studies (14.3)]. Patients received AYVAKIT 25 mg orally once daily with best supportive care (n = 141) or placebo once daily with best supportive care (n = 71).
Serious adverse reactions occurred in 1 patient (0.7%) who received AYVAKIT due to pelvic hematoma.
Permanent discontinuation of AYVAKIT due to an adverse reaction occurred in 1 patient (0.7%) due to dyspnea and dizziness.
Dosage interruptions of AYVAKIT due to an adverse reaction occurred in 5% of patients. Adverse reactions which required dosage interruption included dizziness, blood alkaline phosphatase increased, dyspnea, face edema, pelvic hematoma, liver transaminase increased and respiratory tract infection (1 patient each).
Table 9 summarizes the frequency of adverse reactions in the PIONEER study. The most common adverse reactions (≥ 10%) in the AYVAKIT group were eye edema, dizziness, peripheral edema and flushing. Of all adverse reactions, 55% were Grade 1, 38% were Grade 2 and 7% were Grade 3. Among patients with edema adverse reactions, 95% were Grade 1 and 5% were Grade 2. Among patients with hemorrhage adverse reactions, 86% were Grade 1 and 14% were Grade 2.
Table 9. Adverse Reactions Occurring in AYVAKIT-Treated Patients with Indolent Systemic Mastocytosis During PIONEER Trial Adverse Reactions*,† AYVAKIT (25 mg once daily) + BSC
N=141
%Placebo + BSC
N=71
%Abbreviations: BSC=best supportive care - *
- Adverse reactions that occurred in ≥5% of AYVAKIT-treated patients and ≥2% more than placebo-treated patients.
- †
- Per National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0
- ‡
- Eye edema includes periorbital edema, eye edema, swelling of eyelid, orbital edema, eye swelling, eyelid edema and eyelid ptosis.
- §
- Term includes several similar terms.
- ¶
- Respiratory tract infection includes pneumonia, upper respiratory tract infection, bronchitis and respiratory tract infection.
- #
- Hematoma includes contusion, hematoma and pelvic hematoma.
- Þ
- Hemorrhage includes epistaxis, gingival bleeding, hematochezia, rectal hemorrhage, retinal hemorrhage.
Eye edema‡ 13 7 Dizziness§ 13 10 Peripheral edema§ 12 6 Flushing§ 11 4 Respiratory tract infection¶ 8 1 Face edema 7 1 Rash§ 6 4 Liver transaminase increased§ 6 3 Insomnia 6 3 Hematoma# 6 1 Blood alkaline phosphatase increased 6 1 HemorrhageÞ 5 3 Clinically relevant adverse reactions occurring in <5% of patients were:
Skin and subcutaneous tissue: photosensitivity (2.8%)
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on AYVAKIT - Strong and Moderate CYP3A Inhibitors - Coadministration of AYVAKIT with a strong or moderate CYP3A inhibitor increases avapritinib plasma ...
7.1 Effects of Other Drugs on AYVAKIT
Strong and Moderate CYP3A Inhibitors
Coadministration of AYVAKIT with a strong or moderate CYP3A inhibitor increases avapritinib plasma concentrations [see Clinical Pharmacology (12.3)], which may increase the incidence and severity of adverse reactions of AYVAKIT. Avoid coadministration of AYVAKIT with strong or moderate CYP3A inhibitors. If coadministration of AYVAKIT with a moderate CYP3A inhibitor cannot be avoided, reduce the dose of AYVAKIT [see Dosage and Administration (2.6)].
Strong and Moderate CYP3A Inducers
Coadministration of AYVAKIT with a strong or moderate CYP3A inducer decreases avapritinib plasma concentrations [see Clinical Pharmacology (12.3)], which may decrease efficacy of AYVAKIT. Avoid coadministration of AYVAKIT with strong or moderate CYP3A inducers.
Close7.2 Effects of AYVAKIT on Other Drugs
Coadministration of AYVAKIT with ethinyl estradiol-containing contraceptives may increase the exposure of ethinyl estradiol, which may lead to increased risk of ethinyl estradiol-associated adverse reactions [see Clinical Pharmacology (12.3)].
If the patient is unable to use or tolerate an effective nonhormonal contraceptive or an effective hormonal contraceptive without estrogen, use a formulation of ethinyl estradiol containing 20 mcg or less unless a higher dose is necessary.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], AYVAKIT can cause fetal harm when administered to a ...
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], AYVAKIT can cause fetal harm when administered to a pregnant woman. There are no available data on AYVAKIT use in pregnant women. Oral administration of avapritinib to pregnant rats during the period of organogenesis was teratogenic and embryotoxic at exposure levels approximately 31.4, 6.3 and 2.7 times the human exposure based on AUC at the 25 mg, 200 mg and 300 mg dose, respectively (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In a reproductive toxicity study, administration of avapritinib to rats during the period of organogenesis resulted in decreased fetal body weights, post-implantation loss, and increases in visceral (hydrocephaly, septal defect, and stenosis of the pulmonary trunk) and skeletal (sternum) malformations at doses greater than or equal to 10 mg/kg/day (approximately 31.4, 6.3 and 2.7 times the human exposure based on AUC at the 25 mg, 200 mg and 300 mg dose, respectively).
8.2 Lactation
Risk Summary
There are no data on the presence of avapritinib or its metabolites in human milk or the effects of avapritinib on the breastfed child or milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with AYVAKIT and for 2 weeks following the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating AYVAKIT [see Use in Specific Populations (8.1)].
Contraception
AYVAKIT can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Females
Advise females of reproductive potential to use effective contraception during treatment with AYVAKIT and for 6 weeks after the final dose [see Drug Interactions (7.2)].
Infertility
Females
Based on findings from animal studies, AYVAKIT may impair female fertility. These findings were not reversible within a two month recovery period [see Nonclinical Toxicology (13.1)].
Males
Based on findings from animal studies, AYVAKIT may impair male fertility. These findings were not reversible within a two month recovery period [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of AYVAKIT in pediatric patients have not been established.
8.5 Geriatric Use
Of the 204 patients with unresectable or metastatic GIST who received AYVAKIT in NAVIGATOR, 40% were 65 years or older, while 6% were 75 years and older. Of the 131 patients with AdvSM who received AYVAKIT in EXPLORER and in PATHFINDER, 62% were 65 years or older, while 21% were 75 years and older. Of the 141 patients with ISM who received AYVAKIT in PIONEER, 6% were 65 years or older, while <1% were 75 years and older. No overall differences in safety or efficacy were observed between these patients and younger adult patients.
8.6 Renal Impairment
No dose adjustment is recommended for patients with mild or moderate renal impairment [creatinine clearance (CLcr) 30 to 89 mL/min estimated by Cockcroft-Gault]. The recommended dose of AYVAKIT has not been established for patients with severe renal impairment (CLcr 15 to 29 mL/min) or end-stage renal disease (CLcr <15 mL/min) [see Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
No dose adjustment is recommended for patients with mild [total bilirubin ≤ upper limit of normal (ULN) and aspartate aminotransferase (AST) > ULN or total bilirubin > 1 to 1.5 times ULN and any AST], or moderate [total bilirubin >1.5 to 3 times ULN and any AST] hepatic impairment. Unbound AUC0-INF was 61% higher in subjects with severe hepatic impairment (Child-Pugh Class C) as compared to matched healthy subjects with normal hepatic function. A lower starting dose is recommended in patients with severe hepatic impairment [see Dosage and Administration (2.7)].
-
11 DESCRIPTIONAvapritinib is a kinase inhibitor with the chemical name (S)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1H-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-yl)pyrimidin-5-yl)ethan-1-amine ...
Avapritinib is a kinase inhibitor with the chemical name (S)-1-(4-fluorophenyl)-1-(2-(4-(6-(1-methyl-1H-pyrazol-4-yl)pyrrolo[2,1-f][1,2,4]triazin-4-yl)piperazin-yl)pyrimidin-5-yl)ethan-1-amine. The molecular formula is C26H27FN10, and the molecular weight is 498.57 g/mol. Avapritinib has the following chemical structure:
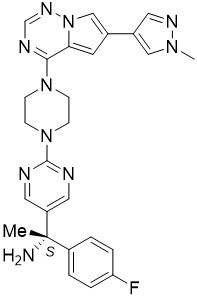
The solubility of avapritinib in 0.1N HCl (pH 1.0) and buffer solutions at pH 2.5, 4.0, and 7.0 (at 25°C) is 3.6 mg/mL, 0.14 mg/mL, 0.07 mg/mL and <0.001 mg/mL respectively, indicating a decrease in solubility with increasing pH.
AYVAKIT (avapritinib) film-coated tablets for oral use are supplied with five strengths that contain 25 mg, 50 mg, 100 mg, 200 mg or 300 mg of avapritinib. The tablets also contain inactive ingredients: copovidone, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose. The tablet coating consists of polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. The blue printing ink, used only for avapritinib 100 mg, 200 mg and 300 mg strength tablets, contains ammonium hydroxide, black iron oxide, esterified shellac, FD&C blue 1, isopropyl alcohol, n-butyl alcohol, propylene glycol, and titanium dioxide.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Avapritinib is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants as well as multiple KIT exon 11, 11/17 and 17 mutants with half ...
12.1 Mechanism of Action
Avapritinib is a tyrosine kinase inhibitor that targets KIT D816V, PDGFRA and PDGFRA D842 mutants as well as multiple KIT exon 11, 11/17 and 17 mutants with half maximal inhibitory concentrations (IC50s) less than 25 nM in biochemical assays. Certain mutations in PDGFRA and KIT can result in the autophosphorylation and constitutive activation of these receptors which can contribute to tumor and mast cell proliferation. Other potential targets for avapritinib include wild type KIT, PDGFRB, and CSFR1.
In cellular assays, avapritinib inhibited the autophosphorylation of KIT D816V with an IC50 of 4 nM, approximately 48-fold lower concentration than wild-type KIT. In cellular assays, avapritinib inhibited the proliferation in KIT mutant cell lines, including a murine mastocytoma cell line and a human mast cell leukemia cell line. Avapritinib also showed growth inhibitory activity in a xenograft model of murine mastocytoma with KIT exon 17 mutation.
Avapritinib inhibited the autophosphorylation of PDGFRA D842V, a mutation associated with resistance to approved kinase inhibitors, with an IC50 of 30 nM. Avapritinib also had anti-tumor activity in mice implanted with an imatinib-resistant patient-derived xenograft model of human GIST with activating KIT exon 11/17 mutations.
12.2 Pharmacodynamics
Exposure-Response Relationships
Gastrointestinal Stromal Tumors or Advanced Systemic Mastocytosis
Based on the data from four clinicals trials conducted in patients with advanced malignancies and AdvSM, including NAVIGATOR, EXPLORER, and PATHFINDER, higher exposure was associated with increased risk of Grade ≥ 3 related adverse effects, any Grade pooled cognitive adverse effects, Grade ≥ 2 pooled cognitive adverse effects, and Grade ≥ 2 pooled edema adverse effects over the dose range of 30 mg to 400 mg (0.1 to 1.33 times the recommended dose for GIST and 0.15 to 2 times the recommended dose for AdvSM) once daily.
Based on exposure and efficacy data from EXPLORER and PATHFINDER (n=84), higher avapritinib exposure was associated with faster time to response over the dose range of 30 mg to 400 mg (0.15 to 2 times the recommended dose for AdvSM) once daily.
Cardiac Electrophysiology
The effect of AYVAKIT on the QTc interval was evaluated in an open-label, single-arm study in 27 patients administered doses of 300 mg or 400 mg (12 to 16 times the lowest recommended 25 mg dose, 1.33 times the highest recommended 300 mg dose) once daily. No large mean increase in QTc (i.e.> 20 ms) was detected at the mean steady state maximum concentration (Cmax) of 899 ng/mL.
Close12.3 Pharmacokinetics
Avapritinib Cmax and AUC increased approximately proportionally over the dose range of 25 mg to 400 mg once daily. Steady state concentrations of avapritinib were reached prior to day 15 following daily dosing. Steady state pharmacokinetic parameters per recommended dosing regimen are described in Table 10.
Table 10. Steady State Pharmacokinetic Parameters of AYVAKIT Following Different Dosing Regimen Pharmacokinetic Parameters 25 mg once daily
(ISM)200 mg once daily
(AdvSM)300 mg once daily
(GIST)Abbreviations: CV%=coefficient of variation Cmax (ng/mL) Geometric Mean (CV%) 70.2 (47.8 %, n=9) 377 (62%, n=18) 813 (52%, n=110) AUC0-24h (h∙ng/mL) Geometric Mean (CV%) 1330 (49.5 %, n = 9) 6600 (54%, n=16) 15400 (48%, n=110) Mean accumulation ratio of AUC 0-24h 4.06 (n=9) 6.41 (n=9) 3.82 (n=34) Absorption
The median time to peak concentration (Tmax) ranged from 2 to 4 hours following single doses of avapritinib.
Distribution
The mean (CV %) apparent volume of distribution of avapritinib is 1310 L (51.5%) at 300 mg in patients with GIST, 1900 L (43.2%) at 200 mg in patients with AdvSM, and 1400 L (59.1%) at 25 mg in patients with ISM. In vitro protein binding of avapritinib is 98.8% and is independent of concentration. The blood-to-plasma ratio is 0.95.
Elimination
The mean plasma elimination half-life of avapritinib was 32 to 57 hours in patients with GIST, 20 to 39 hours in patients with AdvSM, and 38 to 45 hours in patients with ISM. The steady state mean (CV%) apparent oral clearance of avapritinib is 21.8 L/h (54.9%) at 300 mg in patients with GIST, 40.3 L/h (86.0%) at 200 mg in patients with AdvSM, and 21.6 L/h (58.1%) at 25 mg in patients with ISM.
Metabolism
Avapritinib is primarily metabolized by CYP3A4, CYP3A5 and to a lesser extent by CYP2C9 in vitro. Following a single oral dose of approximately 310 mg of radiolabeled avapritinib to healthy subjects, unchanged avapritinib (49%) and its metabolites M690 (hydroxy glucuronide; 35%) and M499 (oxidative deamination; 14%) were the major circulating compounds. The formation of the glucuronide M690 is catalyzed mainly by UGT1A3. Following oral administration of AYVAKIT 300 mg once daily in patients, the steady state AUC of M499 is approximately 80% of the AUC of avapritinib. M499 is not likely to contribute to efficacy at the recommended dose of avapritinib.
Specific Populations
No clinically significant differences in the pharmacokinetics of avapritinib were observed based on age (18 to 90 years), sex, race (White, Black, or Asian), body weight (39.5 to 156.3 kg), mild to moderate (CLcr 30 to 89 mL/min estimated by Cockcroft-Gault) renal impairment, or mild (total bilirubin ≤ ULN and AST > ULN or total bilirubin > 1 to 1.5 times ULN and any AST) to moderate (total bilirubin > 1.5 to 3 times ULN and any AST) hepatic impairment. In a dedicated hepatic impairment study following a single oral dose administration of 100 mg avapritinib, the mean unbound AUC was 61% higher in subjects with severe hepatic impairment (Child-Pugh Class C) as compared to matched healthy subjects with normal hepatic function. The effect of severe renal impairment (CLcr 15 to 29 mL/min) and end-stage renal disease (CLcr < 15 mL/min) on the pharmacokinetics of avapritinib is unknown.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Effect of Strong and Moderate CYP3A Inhibitors on Avapritinib: Coadministration of AYVAKIT 300 mg once daily with itraconazole 200 mg once daily (a strong CYP3A inhibitor) is predicted to increase avapritinib AUC by 600% at steady state.
Coadministration of AYVAKIT 300 mg once daily with fluconazole 200 mg once daily (a moderate CYP3A inhibitor) is predicted to increase avapritinib AUC by 210% at steady state [see Dosage and Administration (2.6), Drug Interactions (7.1)].
Effect of Strong and Moderate CYP3A Inducers on Avapritinib: Coadministration of AYVAKIT 400 mg as a single dose with rifampin 600 mg once daily (a strong CYP3A inducer) decreased avapritinib Cmax by 74% and AUC0-INF by 92%.
Coadministration of AYVAKIT 300 mg once daily with efavirenz 600 mg once daily (a moderate CYP3A inducer) is predicted to decrease avapritinib Cmax by 55% and AUC by 62% at steady state [see Drug Interactions (7.1)].
Effect of Acid-Reducing Agents on Avapritinib: No clinically significant differences in the pharmacokinetics of avapritinib were identified when coadministered with gastric acid reducing agents.
Hormonal Contraceptives: In a drug-drug interaction study of 15 subjects, coadministration of AYVAKIT 25 mg once daily with an oral contraceptive (levonorgestrel 0.15 mg/ethinyl estradiol 0.03 mg) resulted in a mean ethinyl estradiol AUC ratio of 1.15 (90% confidence interval [CI]: 1.04, 1.28) and a mean ethinyl estradiol Cmax ratio of 1.46 (90% CI: 1.17, 1.81) relative to participants administered the oral contraceptive alone. This increase in ethinyl estradiol Cmax may lead to an increased risk of ethinyl estradiol-related adverse events.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: In vitro studies indicate that avapritinib is a time-dependent inhibitor as well as an inducer of CYP3A at clinically relevant concentrations.
Avapritinib is an inhibitor of CYP2C9 at clinically relevant concentrations. Avapritinib is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C19, or CYP2D6 at clinically relevant concentrations.
Avapritinib is not an inducer of CYP1A2 or CYP2B6. Avapritinib is a substrate of CYP3A.
M499 is an inhibitor of CYP3A, CYP2C8, or CYP2C9 at clinically relevant concentrations. M499 is not an inhibitor of CYP1A2, CYP2B6, CYP2C19, or CYP2D6 at clinically relevant concentrations.
Transporter Systems: Avapritinib is an inhibitor of P-glycoprotein (P-gp), intestinal BCRP, MATE1, MATE2-K, and BSEP, but not an inhibitor of OATP1B1, OATP1B3, OAT1, OAT3, OCT1, or OCT2. Avapritinib is not a substrate of P-gp or BCRP, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K and BSEP. The effect of M499 on transporter systems is unknown.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Avapritinib was not mutagenic in a 6-month transgenic mouse study up to the highest dose evaluated at 20 mg/kg/day. Avapritinib was not ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Avapritinib was not mutagenic in a 6-month transgenic mouse study up to the highest dose evaluated at 20 mg/kg/day. Avapritinib was not mutagenic in vitro in the bacterial reverse mutation assay (Ames test). Avapritinib was positive in the in vitro chromosome aberration test in human peripheral blood lymphocytes but negative in the in vivo rat bone marrow micronucleus test, and overall non-genotoxic.
There were no direct effects on fertility in rats of either sex in a dedicated fertility and early embryonic development study. Avapritinib may impair spermatogenesis and adversely affect early embryogenesis. Reduction in sperm production and testicular weight were observed in rats and hypospermatogenesis in dogs administered avapritinib at exposure of 8.7 times and 0.5 time the 300 mg human dose, respectively. Avapritinib partitioned into seminal fluids up to 0.2 times the concentration found in human plasma at 300 mg. In female rats there was an increase in pre-implantation loss at exposure of 5.4 times the human exposure at 300 mg and in early resorptions at exposure 2.7 times the human exposure at 300 mg with an overall decrease in viable embryos. Cystic degeneration of corpora lutea and vaginal mucification was also observed in female rats administered avapritinib for up to 6 months at exposure 1.3 times the human exposure based on AUC at the 300 mg dose.
Close13.2 Animal Toxicology and/or Pharmacology
In repeat dose toxicology studies, administration of avapritinib to rats for up to 28 days and to dogs for up to 3 months resulted in tremors at doses greater than or equal to 100 mg/kg/day or 30 mg/kg/day (approximately 8 and 1.5 times the human exposure based on AUC at the 300 mg dose). Hemorrhage in the brain and spinal cord occurred in dogs at doses greater than or equal to 15 mg/kg/day (approximately 9.0, 1.8, or 0.8 times the human exposure based on AUC at the 25 mg, 200 mg. or 300 mg dose, respectively) and choroid plexus edema in the brain occurred in dogs at doses greater than or equal to 7.5 mg/kg/day (approximately 4.7, 1 or 0.4 times the human exposure based on AUC at the 25 mg, 200 mg or 300 mg dose, respectively), but were not observed in a 9-month study at 5 mg/kg/day.
An in vitro phototoxicity study in 3T3 mouse fibroblasts and an in vivo phototoxicity study in pigmented rats demonstrated that avapritinib has a slight potential for phototoxicity.
-
14 CLINICAL STUDIES14.1 Gastrointestinal Stromal Tumors - The efficacy of AYVAKIT was demonstrated in NAVIGATOR (NCT02508532), a multi-center, single-arm, open-label clinical trial. Eligible patients were required ...
14.1 Gastrointestinal Stromal Tumors
The efficacy of AYVAKIT was demonstrated in NAVIGATOR (NCT02508532), a multi-center, single-arm, open-label clinical trial. Eligible patients were required to have a confirmed diagnosis of GIST and an ECOG performance status (PS) of 0 to 2. Patients received AYVAKIT 300 mg or 400 mg (1.33 times the recommended dose) orally once daily until disease progression or unacceptable toxicity. The trial initially enrolled patients at a starting dose of 400 mg, which was later reduced to the recommended dose of 300 mg due to toxicity. As there was no apparent difference in overall response rate (ORR) between patients who received 300 mg daily compared to those who received 400 mg daily, these patients were pooled for the efficacy evaluation. The major efficacy outcome measure was ORR based on disease assessment by independent radiological review using modified RECIST v1.1 criteria, in which lymph nodes and bone lesions were not target lesions and progressively growing new tumor nodules within a pre-existing tumor mass was progression. An additional efficacy outcome measure was duration of response (DOR).
Patients with GIST Harboring a PDGFRA Exon 18 Mutation
Patients with unresectable or metastatic GIST harboring a PDGFRA exon 18 mutation were identified by local or central assessment using a PCR- or NGS-based assay. The assessment of efficacy was based on a total of 43 patients, including 38 patients with PDGFRA D842V mutations. The median duration of follow up for patients with PDGFRA exon 18 mutations was 10.6 months (range: 0.3 to 24.9 months).
The study population characteristics were median age of 64 years (range: 29 to 90 years), 67% were male, 67% were White, 93% had an ECOG PS of 0-1, 98% had metastatic disease, 53% had largest target lesion >5 cm, and 86% had prior surgical resection. The median number of prior kinase inhibitors was 1 (range: 0 to 5).
Efficacy results in patients with GIST harboring PDGFRA exon 18 mutations including the subgroup of patients with PDGFRA D842V mutations enrolled in NAVIGATOR are summarized in Table 11.
Table 11. Efficacy Results for Patients with GIST Harboring PDGFRA Exon 18 Mutations in NAVIGATOR Efficacy Parameter PDGFRA exon 18*
N = 43PDGFRA D842V
N = 38Abbreviations: CI=confidence interval; NR=not reached Overall Response Rate (95% CI) 84% (69%, 93%) 89% (75%, 97%) Complete Response, n (%) 3 (7%) 3 (8%) Partial Response, n (%) 33 (77%) 31 (82%) Duration of Response n=36 n=34 Median in months (range) NR (1.9†, 20.3†) NR (1.9†, 20.3†) Patients with DOR ≥ 6-months, n (%)‡ 22 (61%) 20 (59%) 14.2 Advanced Systemic Mastocytosis
The efficacy of AYVAKIT was demonstrated in EXPLORER (NCT02561988) and PATHFINDER (NCT03580655), two multi-center, single-arm, open-label clinical trials. Response-evaluable patients include those with a confirmed diagnosis of AdvSM per World Health Organization (WHO) and deemed evaluable by modified international working group-myeloproliferative neoplasms research and treatment-European competence network on mastocytosis (IWG-MRT-ECNM) criteria at baseline as adjudicated by an independent central committee, who received at least 1 dose of AYVAKIT, had at least 2 post-baseline bone marrow assessments, and had been on study for at least 24 weeks, or had an end of study visit. All enrolled patients had an ECOG performance status (PS) of 0 to 3 and 91% had a platelet count of ≥ 50 × 109/L prior to initiation of therapy.
Patients enrolled in EXPLORER received a starting dose of AYVAKIT ranging from 30 mg to 400 mg (0.15 – 2 times the recommended dose) orally once daily. In PATHFINDER, patients were enrolled at a starting dose of 200 mg orally once daily. The efficacy of AYVAKIT in the treatment of AdvSM was based on overall response rate (ORR) in 53 patients with AdvSM dosed at up to 200 mg daily per modified IWG-MRT-ECNM criteria as adjudicated by the central committee. Additional efficacy outcome measures were duration of response (DOR), time to response, and changes in individual measures of mast cell burden.
The median duration of follow up for these patients was 11.6 months (95% confidence interval: 9.9, 16.3).
The study population characteristics were median age of 67 years (range: 37 to 85 years), 58% were male, 98% were White, 68% had an ECOG PS of 0-1, 32% had an ECOG PS of 2-3, 40% had ongoing corticosteroid therapy use for AdvSM at baseline, 66% had prior antineoplastic therapy, 47% had received prior midostaurin, and 94% had a D816V mutation. The median bone marrow mast cell infiltrate was 50%, the median serum tryptase level was 255.8 ng/mL, and the median KIT D816V mutant allele fraction was 12.2%.
Efficacy results in patients with AdvSM enrolled in EXPLORER and PATHFINDER are summarized in Table 12.
Table 12. Efficacy Results for Patients with AdvSM in EXPLORER and PATHFINDER All evaluable patients ASM SM-AHN MCL Abbreviations: CI=confidence interval; CR=complete remission; CRh=complete remission with partial recovery of peripheral blood counts; PR=partial remission Overall Response Rate*, %
per modified IWG-MRT-ECNM
(95% CI†)N=53
57
(42, 70)N=2
100
(16, 100)N=40
58
(41, 73)N=11
45
(17, 77)Complete Remission with full or partial hematologic recovery, % 28 50 33 9 Partial Remission, % 28 50 25 36 Clinical Improvement, % 15 0 20 0 Stable Disease, % 19 0 13 45 For all evaluable patients, the median duration of response was 38.3 months (95% confidence interval: 19, not estimable) and the median time to response was 2.1 months.
In the subgroup of patients with MCL, the efficacy of AYVAKIT was based on complete remission (CR).
Close14.3 Indolent Systemic Mastocytosis
The efficacy of AYVAKIT was demonstrated in PIONEER (NCT03731260), a randomized, double-blind, placebo-controlled trial conducted in adult patients with Indolent Systemic Mastocytosis (ISM) based on World Health Organization (WHO) classification. Enrolled patients had moderate to severe symptoms despite receiving at least 2 symptom directed therapies. Patients were randomized to receive 25 mg AYVAKIT orally once daily with best supportive care versus placebo with best supportive care. The treatment duration was over a 24-week period, during the randomized portion of the study.
Efficacy was based on the absolute mean change from baseline to Week 24 in the Indolent Systemic Mastocytosis-Symptom Assessment Form (ISM-SAF) total symptom score (TSS). The ISM-SAF is a patient-reported outcome measure assessing ISM signs and symptoms: abdominal pain, nausea, diarrhea, spots, itching, flushing, bone pain, fatigue, dizziness, headache, brain fog. Scores ranged from 0 ("none") to 10 ("worst imaginable"). The item scores were summed to calculate a daily ISM-SAF TSS (range 0-110), with higher scores indicating greater symptom severity. A biweekly average ISM-SAF TSS was used to evaluate efficacy endpoints.
Additional supportive results included the proportion of AYVAKIT-treated patients achieving ≥50% reduction from baseline through Week 24 in TSS compared to placebo. Objective measures of mast cell burden were assessed including the proportion of AYVAKIT-treated patients with a ≥50% reduction from baseline through Week 24 in serum tryptase, peripheral blood KIT D816V allele fraction and bone marrow mast cells.
The median age of the patients who received AYVAKIT was 50 years (range: 18 to 77 years), 71% were female, 77% were White, <1% were Asian, 3% had other race and 19% had missing race. Ethnicities included 4% Hispanic or Latino. KIT D816V mutations were identified in 93% of patients. At baseline, the mean TSS was 50.17 (standard deviation: 19.15), the median serum tryptase level was 38.40 ng/mL, the median KIT D816V mutant allele fraction was 0.39% by ddPCR and the median bone marrow mast cell infiltrate was 7%. Study population characteristics were similar in the placebo group.
The majority of patients who received AYVAKIT (99.3%) or placebo (100%) received concomitant best supportive care at baseline (median of 3 therapies in the AYVAKIT group and 4 in the placebo group). The most common therapies in the AYVAKIT group were H1 antihistamines (97%), H2 antihistamines (66%), leukotriene inhibitors (35%) and cromolyn sodium (30%).
Efficacy results are summarized in Tables 13 and 14.
Table 13. Efficacy Results for Patients with ISM in PIONEER at Week 24 Efficacy Parameter AYVAKIT (25 mg once daily) + BSC
N=141Placebo + BSC
N=712-sided p-value Abbreviations: BSC=best supportive care; CI=confidence interval; ISM-SAF= Indolent Systemic Mastocytosis-Symptom Assessment Form TSS=Total Symptom Score Absolute Mean change in the ISM-SAF TSS* Change from baseline (95% CI) -15.33
(-18.36, -12.31)-9.64
(-13.61, -5.68)0.012 Difference from placebo (95% CI) -5.69
(-10.16, -1.23)% of patients achieving ≥50% reduction in the ISM-SAF TSS† (95% CI) 25
(17.9, 32.8)10
(4.1, 19.3)0.009 Table 14. Efficacy Results Related to Mast Cell Burden for Patients with ISM in PIONEER at Week 24 Efficacy Parameter AYVAKIT (25 mg once daily) + BSC Placebo + BSC 2-sided p-value Abbreviations: BSC=best supportive care; CI=confidence interval % of patients with a ≥50% reduction in serum tryptase (95% CI) N=141
53.9
(45.3, 62.3)N=71
0
(0.0, 5.1)<0.0001 % of patients with a ≥50% reduction in peripheral blood KIT D816V allele fraction or undetectable (95% CI) N=118
67.8
(58.6, 76.1)N=63
6.3
(1.8, 15.5)<0.0001 % of patients with a ≥50% reduction in bone marrow mast cells or no aggregates (95% CI) N=106
52.8
(42.9, 62.6)N=57
22.8
(12.7, 35.8)<0.0001 To aid in the interpretation of the ISM-SAF TSS absolute mean change from baseline results, the proportion of patients reporting less than or equal to any particular level of change in the ISM-SAF TSS from baseline to Week 24 is depicted in a cumulative distribution function plot as shown in Figure 1.
Figure 1: Cumulative Proportion of Patients with ISM in PIONEER Reporting Change in ISM-SAF TSS From Baseline to Week 24

-
16 HOW SUPPLIED/STORAGE AND HANDLINGAYVAKIT (avapritinib) tablets are supplied as follows: 25 mg, round, white film-coated tablet with debossed text. One side reads "BLU" and the other side reads "25"; available in bottles of 30 ...
AYVAKIT (avapritinib) tablets are supplied as follows:
25 mg, round, white film-coated tablet with debossed text. One side reads "BLU" and the other side reads "25"; available in bottles of 30 tablets (NDC 72064-125-30).
50 mg, round, white film-coated tablet with debossed text. One side reads "BLU" and the other side reads "50"; available in bottles of 30 tablets (NDC 72064-150-30).
100 mg, round, white film-coated tablet, printed with blue ink "BLU" on one side and "100" on the other side; available in bottles of 30 tablets (NDC 72064-110-30).
200 mg, capsule shaped, white film-coated tablet, printed with blue ink "BLU" on one side and "200" on the other side; available in bottles of 30 tablets (NDC 72064-120-30).
300 mg, capsule shaped, white film-coated tablet, printed with blue ink "BLU" on one side and "300" on the other side; available in bottles of 30 tablets (NDC 72064-130-30).
CloseStore at 20°C to 25°C (68°F to 77°F); excursions are permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Intracranial Hemorrhage - Advise patients to contact their healthcare provider immediately if experiencing ...
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Intracranial Hemorrhage
Advise patients to contact their healthcare provider immediately if experiencing neurological signs and symptoms that may be associated with intracranial hemorrhage (i.e., severe headache, vomiting, drowsiness, dizziness, confusion, slurred speech, or paralysis) [see Warnings and Precautions (5.1)].
Inform patients with AdvSM of the need to monitor platelet counts before and during treatment [see Warnings and Precautions (5.1)].
Cognitive Effects
Advise patients and caretakers to notify their healthcare provider if they experience new or worsening cognitive symptoms. Advise patients not to drive or operate hazardous machinery if they are experiencing cognitive adverse reactions [see Warnings and Precautions (5.2)].
Photosensitivity
Inform patients that there is a potential risk of photosensitivity reactions with AYVAKIT. Advise patients to limit direct ultraviolet exposure by using sunscreen and protective clothing during treatment with AYVAKIT [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment with AYVAKIT and for 6 weeks after the final dose [see Drug Interactions (7.2), Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with AYVAKIT and for 6 weeks after the final dose [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with AYVAKIT and for 2 weeks following the final dose [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that AYVAKIT may impair fertility at 200 mg or 300 mg [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)]. Advise males of reproductive potential that AYVAKIT may decrease sperm production at 200 mg or 300 mg [see Use in Specific Populations (8.3), Nonclinical Toxicology (13.1)].
Drug Interactions
Advise patients and caregivers to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7.1)].
CloseAdministration
Advise patients to take AYVAKIT on an empty stomach, at least 1 hour before or at least 2 hours after a meal [see Dosage and Administration (2.1)].
-
SPL UNCLASSIFIED SECTIONManufactured for: Blueprint Medicines Corporation, Cambridge, MA 02139, USA
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: November/2024 PATIENT INFORMATION - AYVAKIT® (aye vah ...Close
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: November/2024 PATIENT INFORMATION
AYVAKIT® (aye vah kit)
(avapritinib)
tablets, for oral useWhat is AYVAKIT?
AYVAKIT is a prescription medicine used to treat adults with:
- a certain type of stomach, bowel, or esophagus cancer called gastrointestinal stromal tumor (GIST) that cannot be treated with surgery or that has spread to other parts of the body (metastatic), and that is caused by certain abnormal platelet-derived growth factor receptor alpha (PDGFRA) genes. Your healthcare provider will perform a test to make sure that you have this abnormal PDGFRA gene and that AYVAKIT is right for you.
- advanced systemic mastocytosis (AdvSM), including aggressive systemic mastocytosis (ASM), systemic mastocytosis with an associated hematological neoplasm (SM-AHN), and mast cell leukemia (MCL).
AYVAKIT is not recommended for the treatment of AdvSM in people with low platelet counts (less than 50 × 109/L). - indolent systemic mastocytosis (ISM).
AYVAKIT is not recommended for the treatment of ISM in people with low platelet counts (less than 50 × 109/L).
Before taking AYVAKIT, tell your healthcare provider about all of your medical conditions, including if you: - have a history of bulging or weakening of a blood vessel wall (aneurysm) or bleeding in your brain
- history of stroke within the last year
- have low platelet counts
- have or have had liver problems
- are pregnant or plan to become pregnant. AYVAKIT can cause harm to your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with AYVAKIT.
- You should use effective birth control (contraception) during treatment with AYVAKIT and for 6 weeks after the final dose of AYVAKIT. Talk to your healthcare provider about birth control methods that may be right for you.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with AYVAKIT.
Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment and for 6 weeks after the final dose of AYVAKIT. - are breastfeeding or plan to breastfeed. It is not known if AYVAKIT passes into your breast milk. Do not breastfeed during treatment with AYVAKIT and for at least 2 weeks after the final dose of AYVAKIT. Talk to your healthcare provider about the best way to feed your baby during this time.
Especially tell your healthcare provider if you take:- estrogen-containing hormonal birth control (contraception)
- medicines that prevent blood clots
How should I take AYVAKIT? - Take AYVAKIT exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking AYVAKIT unless your healthcare provider tells you to.
- AYVAKIT is usually taken 1 time each day.
- Take AYVAKIT tablet(s) on an empty stomach at least 1 hour before or at least 2 hours after a meal.
- If you miss a dose of AYVAKIT, take it as soon as you remember unless your next scheduled dose is due within 8 hours. Take the next dose at your regular time.
- If you vomit after taking a dose of AYVAKIT, do not take an extra dose. Take your next dose at your next scheduled time.
What should I avoid while taking AYVAKIT? - Do not drive or operate heavy machinery if you have confusion or trouble thinking during treatment with AYVAKIT.
- Your skin may be sensitive to the sun or other forms of light (photosensitivity) during treatment with AYVAKIT. Avoid or limit exposure to direct sunlight, sunlamps, and other sources of ultraviolet radiation during treatment and for 1 week after stopping treatment with AYVAKIT. Use sunscreen or wear clothes that cover your skin if you need to be out in the sun.
What are the possible side effects of AYVAKIT?
AYVAKIT may cause serious side effects, including:-
Bleeding in your brain. Serious bleeding in the brain may happen during treatment with AYVAKIT and may lead to death. Stop taking AYVAKIT and tell your healthcare provider right away if you develop any symptoms such as severe headache, nausea, vomiting, vision changes, drowsiness, dizziness, confusion, or severe weakness on one or more side of your body. Bleeding in the brain has not been seen in people treated with AYVAKIT for ISM.
If you have AdvSM, your healthcare provider will check your platelet counts before and during treatment with AYVAKIT. - Cognitive effects. Cognitive side effects can happen during treatment with AYVAKIT and can be severe. Tell your healthcare provider if you develop any new or worsening cognitive symptoms including:
- forgetfulness
- confusion
- getting lost
- trouble thinking
- drowsiness
- trouble staying awake (somnolence)
- word finding problems
- seeing objects or hearing things that are not there (hallucinations)
- change in mood or behavior
- Skin sensitivity to sunlight (photosensitivity). See "What should I avoid while taking AYVAKIT?"
The most common side effects of AYVAKIT in people with GIST include: - fluid retention or swelling
- nausea
- tiredness or weakness
- trouble thinking
- vomiting
- decreased appetite
- diarrhea
- increased eye tearing
- stomach area (abdominal) pain
- constipation
- rash
- dizziness
- hair color changes
- changes in certain blood tests
The most common side effects of AYVAKIT in people with AdvSM include: - fluid retention or swelling
- diarrhea
- nausea
- tiredness or weakness
- changes in certain blood tests
The most common side effects of AYVAKIT in people with ISM include: - swelling around your eyes
- dizziness
- swelling of your arms and legs
- flushing
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with AYVAKIT if you develop certain side effects.
AYVAKIT may cause fertility problems in females and males. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of AYVAKIT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store AYVAKIT? - Store AYVAKIT tablets at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of AYVAKIT.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not take AYVAKIT for a condition for which it was not prescribed. Do not give AYVAKIT to other people, even if they have the same condition that you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about AYVAKIT that is written for health professionals.What are the ingredients in AYVAKIT?
Active ingredient: avapritinib
Inactive ingredients: copovidone, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose.
Film coat: polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Blue printing ink (100 mg, 200 mg and 300 mg tablets only): ammonium hydroxide, black iron oxide, esterified shellac, FD&C blue 1, isopropyl alcohol, n-butyl alcohol, propylene glycol, and titanium dioxide.
Manufactured for: Blueprint Medicines Corporation, Cambridge, MA 02139, USA
© 2023 Blueprint Medicines Corporation. All rights reserved.
For more information, go to www.AYVAKIT.com or call 1-888-258-7768. -
PRINCIPAL DISPLAY PANEL - 100 mg Bottle LabelNDC 72064-110-30 - 30 Tablets - AYVAKIT™ (avapritinib) tablets - 100 mg - For Oral Use - Rx only
-
PRINCIPAL DISPLAY PANEL - 200 mg Bottle LabelNDC 72064-120-30 - 30 Tablets - AYVAKIT™ (avapritinib) tablets - 200 mg - For Oral Use - Rx only
-
PRINCIPAL DISPLAY PANEL - 300 mg Bottle LabelNDC 72064-130-30 - 30 Tablets - AYVAKIT™ (avapritinib) tablets - 300 mg - For Oral Use - Rx only
-
PRINCIPAL DISPLAY PANEL - 25 mg Tablet Bottle LabelNDC 72064-125-30 - 30 Tablets - AYVAKIT™ (avapritinib) tablets - 25 mg - For Oral Use - Rx only
-
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle LabelNDC 72064-150-30 - 30 Tablets - AYVAKIT™ (avapritinib) tablets - 50 mg - For Oral Use - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information
AYVAKIT avapritinib tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72064-110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength avapritinib (UNII: 513P80B4YJ) (avapritinib - UNII:513P80B4YJ) avapritinib 100 mg Inactive Ingredients Ingredient Name Strength Microcrystalline cellulose 101 (UNII: 7T9FYH5QMK) Microcrystalline cellulose (UNII: OP1R32D61U) Copovidone K25-31 (UNII: D9C330MD8B) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Talc (UNII: 7SEV7J4R1U) Polyethylene Glycol 4000 (UNII: 4R4HFI6D95) Polyvinyl Alcohol (130000 MW) (UNII: 660SZ0AKDA) Titanium Dioxide (UNII: 15FIX9V2JP) Shellac (UNII: 46N107B71O) Butyl Alcohol (UNII: 8PJ61P6TS3) FD&C Blue No. 1 (UNII: H3R47K3TBD) Isopropyl Alcohol (UNII: ND2M416302) Ferrosoferric Oxide (UNII: XM0M87F357) Propylene Glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) Product Characteristics Color WHITE Score no score Shape ROUND Size 9mm Flavor Imprint Code BLU;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72064-110-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212608 01/09/2020 AYVAKIT avapritinib tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72064-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength avapritinib (UNII: 513P80B4YJ) (avapritinib - UNII:513P80B4YJ) avapritinib 200 mg Inactive Ingredients Ingredient Name Strength Microcrystalline cellulose 101 (UNII: 7T9FYH5QMK) Microcrystalline cellulose (UNII: OP1R32D61U) Copovidone K25-31 (UNII: D9C330MD8B) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Talc (UNII: 7SEV7J4R1U) Polyethylene Glycol 4000 (UNII: 4R4HFI6D95) Polyvinyl Alcohol (130000 MW) (UNII: 660SZ0AKDA) Titanium Dioxide (UNII: 15FIX9V2JP) Shellac (UNII: 46N107B71O) Butyl Alcohol (UNII: 8PJ61P6TS3) FD&C Blue No. 1 (UNII: H3R47K3TBD) Isopropyl Alcohol (UNII: ND2M416302) Ferrosoferric Oxide (UNII: XM0M87F357) Propylene Glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) Product Characteristics Color WHITE Score no score Shape OVAL Size 16mm Flavor Imprint Code BLU;200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72064-120-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212608 01/09/2020 AYVAKIT avapritinib tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72064-130 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength avapritinib (UNII: 513P80B4YJ) (avapritinib - UNII:513P80B4YJ) avapritinib 300 mg Inactive Ingredients Ingredient Name Strength Microcrystalline cellulose 101 (UNII: 7T9FYH5QMK) Microcrystalline cellulose (UNII: OP1R32D61U) Copovidone K25-31 (UNII: D9C330MD8B) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Talc (UNII: 7SEV7J4R1U) Polyethylene Glycol 4000 (UNII: 4R4HFI6D95) Polyvinyl Alcohol (130000 MW) (UNII: 660SZ0AKDA) Titanium Dioxide (UNII: 15FIX9V2JP) Shellac (UNII: 46N107B71O) Butyl Alcohol (UNII: 8PJ61P6TS3) FD&C Blue No. 1 (UNII: H3R47K3TBD) Isopropyl Alcohol (UNII: ND2M416302) Ferrosoferric Oxide (UNII: XM0M87F357) Propylene Glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) Product Characteristics Color WHITE Score no score Shape OVAL Size 18mm Flavor Imprint Code BLU;300 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72064-130-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/09/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212608 01/09/2020 AYVAKIT avapritinib tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72064-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength avapritinib (UNII: 513P80B4YJ) (avapritinib - UNII:513P80B4YJ) avapritinib 25 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Copovidone K25-31 (UNII: D9C330MD8B) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Talc (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) Polyvinyl Alcohol (130000 MW) (UNII: 660SZ0AKDA) Titanium Dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND Size 5mm Flavor Imprint Code BLU;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72064-125-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/16/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212608 06/16/2021 AYVAKIT avapritinib tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72064-150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength avapritinib (UNII: 513P80B4YJ) (avapritinib - UNII:513P80B4YJ) avapritinib 50 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Copovidone K25-31 (UNII: D9C330MD8B) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Talc (UNII: 7SEV7J4R1U) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) Polyvinyl Alcohol (130000 MW) (UNII: 660SZ0AKDA) Titanium Dioxide (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code BLU;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72064-150-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/16/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212608 06/16/2021
CloseLabeler - Blueprint Medicines Corporation (021905363)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
AYVAKIT- avapritinib tablet, film coated
Number of versions: 9
RxNorm
AYVAKIT- avapritinib tablet, film coated
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2272112 | avapritinib 100 MG Oral Tablet | PSN |
| 2 | 2272112 | avapritinib 100 MG Oral Tablet | SCD |
| 3 | 2272118 | AYVAKIT 100 MG Oral Tablet | PSN |
| 4 | 2272118 | avapritinib 100 MG Oral Tablet [Ayvakit] | SBD |
| 5 | 2272118 | Ayvakit 100 MG Oral Tablet | SY |
| 6 | 2272120 | avapritinib 200 MG Oral Tablet | PSN |
| 7 | 2272120 | avapritinib 200 MG Oral Tablet | SCD |
| 8 | 2272122 | AYVAKIT 200 MG Oral Tablet | PSN |
| 9 | 2272122 | avapritinib 200 MG Oral Tablet [Ayvakit] | SBD |
| 10 | 2272122 | Ayvakit 200 MG Oral Tablet | SY |
| 11 | 2272124 | avapritinib 300 MG Oral Tablet | PSN |
| 12 | 2272124 | avapritinib 300 MG Oral Tablet | SCD |
| 13 | 2272126 | AYVAKIT 300 MG Oral Tablet | PSN |
| 14 | 2272126 | avapritinib 300 MG Oral Tablet [Ayvakit] | SBD |
| 15 | 2272126 | Ayvakit 300 MG Oral Tablet | SY |
| 16 | 2559723 | avapritinib 25 MG Oral Tablet | PSN |
| 17 | 2559723 | avapritinib 25 MG Oral Tablet | SCD |
| 18 | 2559725 | AYVAKIT 25 MG Oral Tablet | PSN |
| 19 | 2559725 | avapritinib 25 MG Oral Tablet [Ayvakit] | SBD |
| 20 | 2559725 | Ayvakit 25 MG Oral Tablet | SY |
| 21 | 2559727 | avapritinib 50 MG Oral Tablet | PSN |
| 22 | 2559727 | avapritinib 50 MG Oral Tablet | SCD |
| 23 | 2559729 | AYVAKIT 50 MG Oral Tablet | PSN |
| 24 | 2559729 | avapritinib 50 MG Oral Tablet [Ayvakit] | SBD |
| 25 | 2559729 | Ayvakit 50 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
AYVAKIT- avapritinib tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=645c887c-8cd4-4623-8da9-ac223d71a8b9
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
AYVAKIT- avapritinib tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 72064-110-30 |
| 2 | 72064-120-30 |
| 3 | 72064-125-30 |
| 4 | 72064-130-30 |
| 5 | 72064-150-30 |










