Label: ASPARLAS- calaspargase pegol injection, solution
- NDC Code(s): 72694-515-01
- Packager: Servier Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ASPARLAS safely and effectively. See full prescribing information for ASPARLAS. ASPARLAS® (calaspargase pegol-mknl) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Acute Lymphoblastic Leukemia - ASPARLAS is indicated as a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia in pediatric and young adult ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dose of ASPARLAS is 2,500 units/m2 given intravenously no more frequently than every 21 days. 2.2 Recommended Premedication - Premedicate patients ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 3,750 units/5 mL (750 units/mL) clear, colorless solution in a single-dose vial.
-
4 CONTRAINDICATIONSASPARLAS is contraindicated in patients with: History of serious hypersensitivity reactions, including anaphylaxis, to pegylated L-asparaginase therapy [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Grade 3 and 4 hypersensitivity reactions, including anaphylaxis, have been reported in clinical trials with ASPARLAS with an incidence between 7 and 21% [see ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity [see Warnings and Precautions (5.1)] Pancreatitis [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on published literature studies with L-asparaginase in pregnant animals, ASPARLAS can cause fetal harm when administered to a pregnant woman. There are no ...
-
11 DESCRIPTIONCalaspargase pegol-mknl contains an asparagine specific enzyme derived from Escherichia coli, as a conjugate of L-asparaginase (L-asparagine amidohydrolase) and monomethoxypolyethylene glycol ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - L-asparaginase is an enzyme that catalyzes the conversion of the amino acid L-asparagine into aspartic acid and ammonia. The pharmacological effect of ASPARLAS is ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity, mutagenicity, and impairment of fertility studies have not been conducted with calaspargase pegol-mknl.
-
14 CLINICAL STUDIES14.1 Acute Lymphoblastic Leukemia - The determination of efficacy was based on a demonstration of the achievement and maintenance of nadir serum asparaginase activity (NSAA) above the level of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGASPARLAS (calaspargase pegol-mknl) injection is supplied as a clear, colorless, preservative-free sterile solution in a single-dose vial containing 3,750 units of calaspargase pegol-mknl per 5 mL ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity - Inform patients on the possibility of serious allergic reactions, including anaphylaxis. Instruct the patient on the symptoms of allergic reactions and to seek medical advice ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Servier Pharmaceuticals LLC - Boston, MA 02210 - U.S. License No. 2125 - ASPARLAS is a registered trademark of Servier IP UK Ltd, a wholly owned, indirect subsidiary of Les Laboratoires ...
-
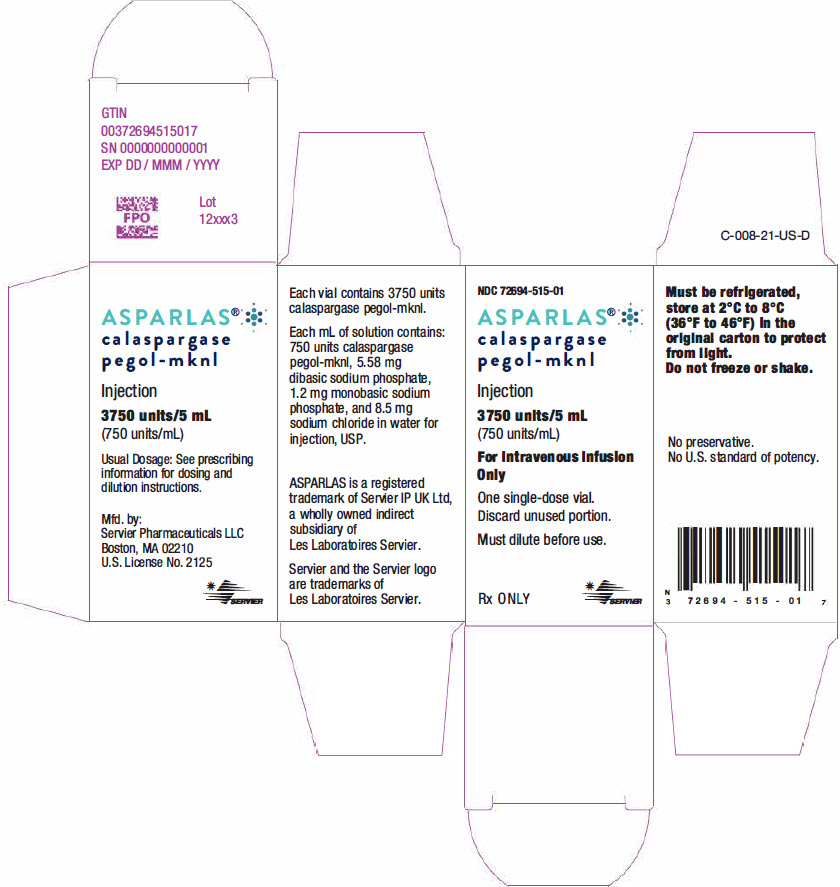
PRINCIPAL DISPLAY PANEL - 5 mL Vial CartonNDC 72694-515-01 - ASPARLAS® calaspargase - pegol-mknl - Injection - 3750 units/5 mL - (750 units/mL) For Intravenous Infusion - Only - One single-dose vial. Discard unused portion. Must dilute before use. Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information