Label: ASCLERA- polidocanol injection, solution
- NDC Code(s): 67850-140-05, 67850-141-05
- Packager: Methapharm, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Asclera® Injection safely and effectively. See full prescribing information for Asclera. Asclera (polidocanol) Injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAsclera® (polidocanol) is indicated to sclerose uncomplicated spider veins (varicose veins ≤1 mm in diameter) and uncomplicated reticular veins (varicose veins 1 to 3 mm in diameter) in the lower ...
-
2 DOSAGE AND ADMINISTRATIONFor intravenous use only. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use ...
-
3 DOSAGE FORMS AND STRENGTHSAsclera is available as a 0.5% and 1% solution in 2 mL glass ampules.
-
4 CONTRAINDICATIONSAsclera is contraindicated for patients with known allergy to polidocanol and patients with acute thromboembolic diseases.
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylaxis - Severe allergic reactions have been reported following polidocanol use, including anaphylactic reactions, some of them fatal. Severe reactions are more frequent with use of ...
-
6 ADVERSE REACTIONS6.1 Clinical Study Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo drug-drug interactions have been studied with Asclera.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data from case reports on use of polidocanol-containing products, including ASCLERA, in pregnant women have not identified any drug-associated risk ...
-
10 OVERDOSAGEOverdose may result in a higher incidence of localized reactions such as necrosis.
-
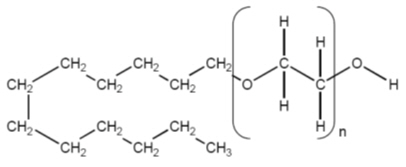
11 DESCRIPTIONAsclera is a sterile, nonpyrogenic, and colorless to faintly greenish-yellow solution of polidocanol for intravenous use as a sclerosing agent. The active ingredient, polidocanol is a non-ionic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The active ingredient of Asclera is polidocanol. Polidocanol is a sclerosing agent that locally damages the endothelium of blood vessels. When injected intravenously ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate carcinogenic potential have not been conducted with polidocanol. Polidocanol was negative in bacterial ...
-
14 CLINICAL STUDIESAsclera was evaluated in a multicenter, randomized, double-blind, placebo- and comparator-controlled trial (EASI-study) in patients with spider or reticular varicose veins. A total of 338 patients ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Asclera is supplied in single-use, preservative free ampules in the following packages: NDC 67850-140-05 Five 0.5% ampules (2 mL) NDC 67850-141-05 Five 1.0% ampules (2 mL) Each ampule is intended ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to wear compression stockings or support hose on the treated legs continuously for 2 to 3 days and for 2 to 3 weeks during the daytime. Compression stockings or support hose ...
-
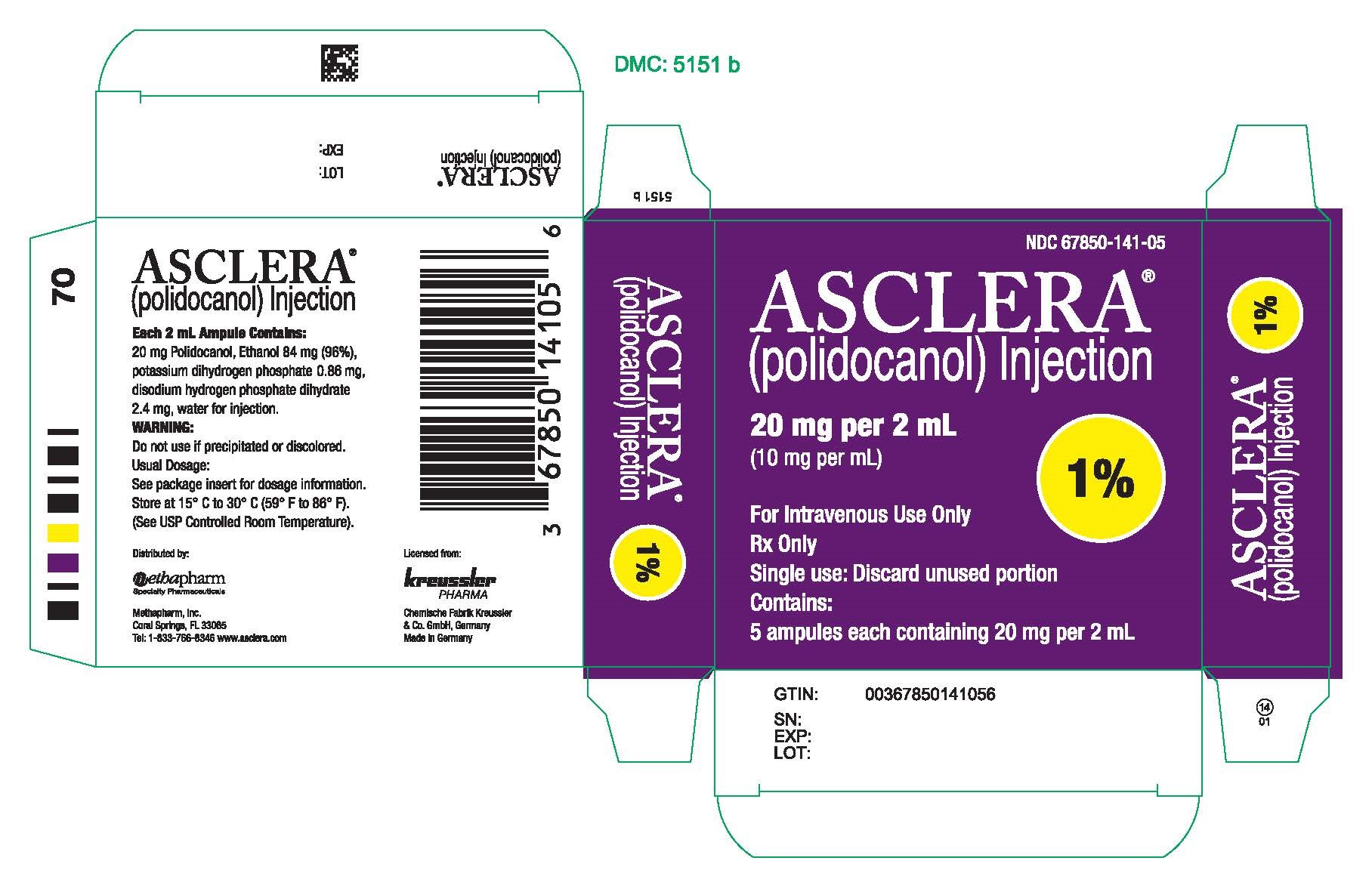
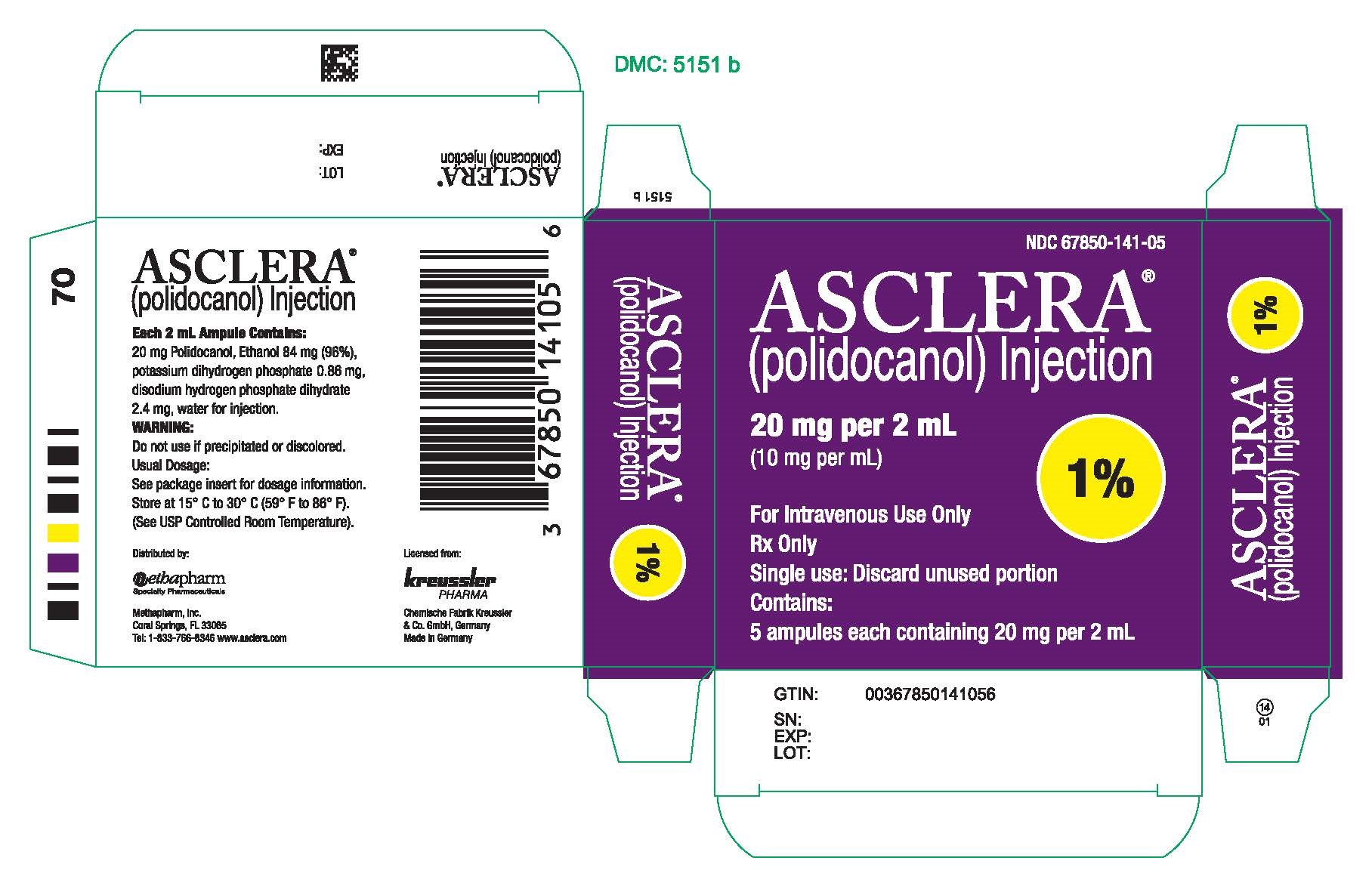
PRINCIPAL DISPLAY PANEL - 20 mg Ampule CartonNDC 67850-141-05 - ASCLERA® (polidocanol) Injection - 20 mg per 2 mL - (10 mg per mL) 1% For Intravenous Use Only - Rx Only - Single use: Discard unused portion - Contains: 5 ampules each containing ...
-
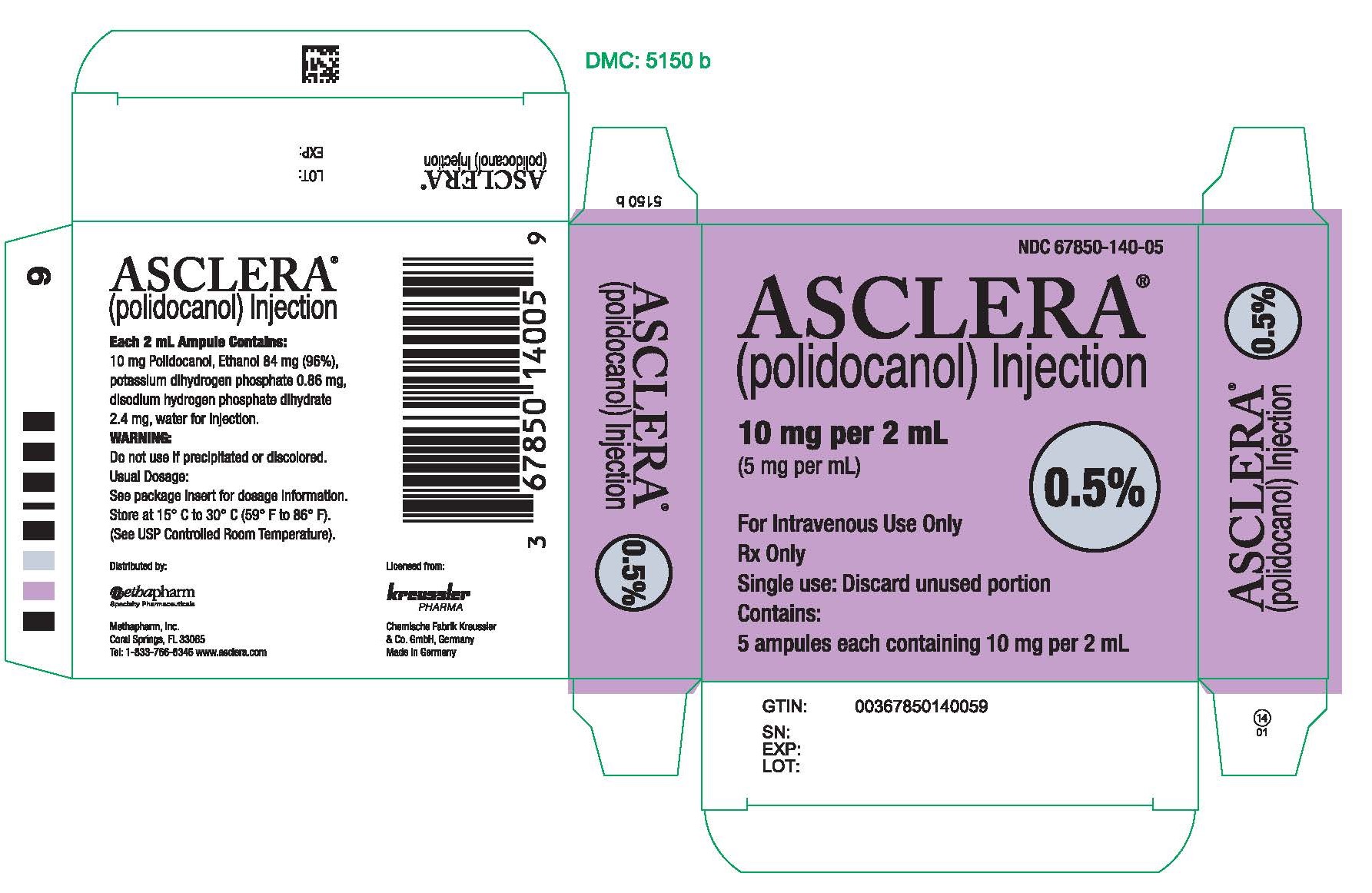
PRINCIPAL DISPLAY PANEL - 10 mg Ampule CartonNDC 67850-140-05 - ASCLERA® (polidocanol) Injection - 10 mg per 2 mL - (5 mg per mL) 0.5% For Intravenous Use Only - Rx Only - Single use: Discard unused portion - Contains: 5 ampules each containing ...
-
INGREDIENTS AND APPEARANCEProduct Information