Label: ARZERRA- ofatumumab injection, solution
- NDC Code(s): 0078-0669-13, 0078-0669-61, 0078-0690-61
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARZERRA safely and effectively. See full prescribing information for ARZERRA. ARZERRA® (ofatumumab) injection, for intravenous ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HEPATITIS B VIRUS REACTIVATION AND PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
- Hepatitis B Virus (HBV) reactivation can occur in patients receiving CD20-directed cytolytic antibodies, including ARZERRA®, in some cases resulting in fulminant hepatitis, hepatic failure, and death [see Warnings and Precautions (5.2)].
- Progressive Multifocal Leukoencephalopathy (PML) resulting in death can occur in patients receiving CD20-directed cytolytic antibodies, including ARZERRA [see Warnings and Precautions (5.4)].
-
1 INDICATIONS AND USAGEChronic Lymphocytic Leukemia (CLL) ARZERRA (ofatumumab) is indicated: in combination with chlorambucil, for the treatment of previously untreated patients with CLL for whom ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage Regimen - Dilute and administer as an intravenous infusion according to the following schedules. Do not administer as an intravenous push or bolus or as a subcutaneous ...
-
3 DOSAGE FORMS AND STRENGTHS100 mg/5 mL single‑use vial for intravenous infusion. 1,000 mg/50 mL single‑use vial for intravenous infusion.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion Reactions - ARZERRA can cause serious, including fatal, infusion reactions manifesting as bronchospasm, dyspnea, laryngeal edema, pulmonary edema, flushing, hypertension ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in greater detail in other sections of the labeling: Infusion Reactions [see Warnings and Precautions (5.1)] Hepatitis B Virus Reactivation ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - ARZERRA may cause fetal B-cell depletion based on findings from animal studies and the drug’s mechanism of action [see Clinical Pharmacology (12.1)]. There are ...
-
11 DESCRIPTIONARZERRA (ofatumumab) is an IgG1κ human monoclonal antibody with a molecular weight of approximately 149 kDa. The antibody was generated via transgenic mouse and hybridoma technology and is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ofatumumab binds specifically to both the small and large extracellular loops of the CD20 molecule. The CD20 molecule is expressed on normal B lymphocytes (pre–B ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or mutagenicity studies of ofatumumab have been conducted. In a repeat-dose toxicity study, no tumorigenic or ...
-
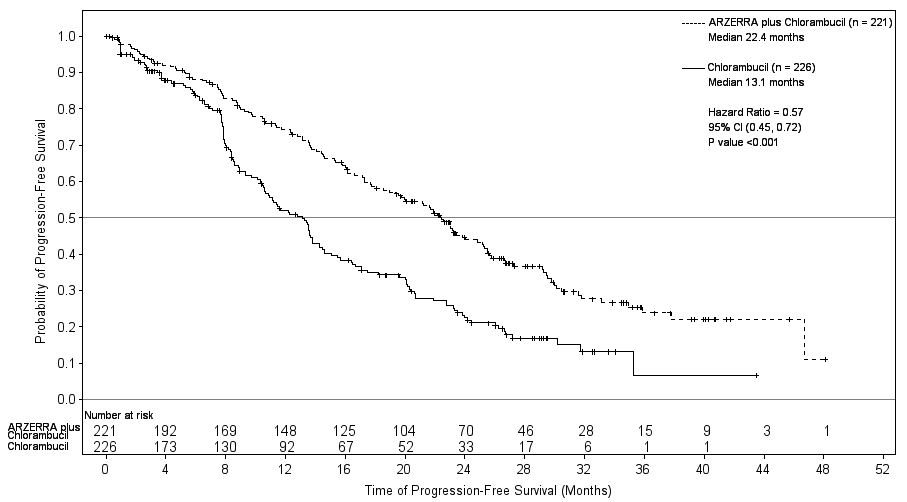
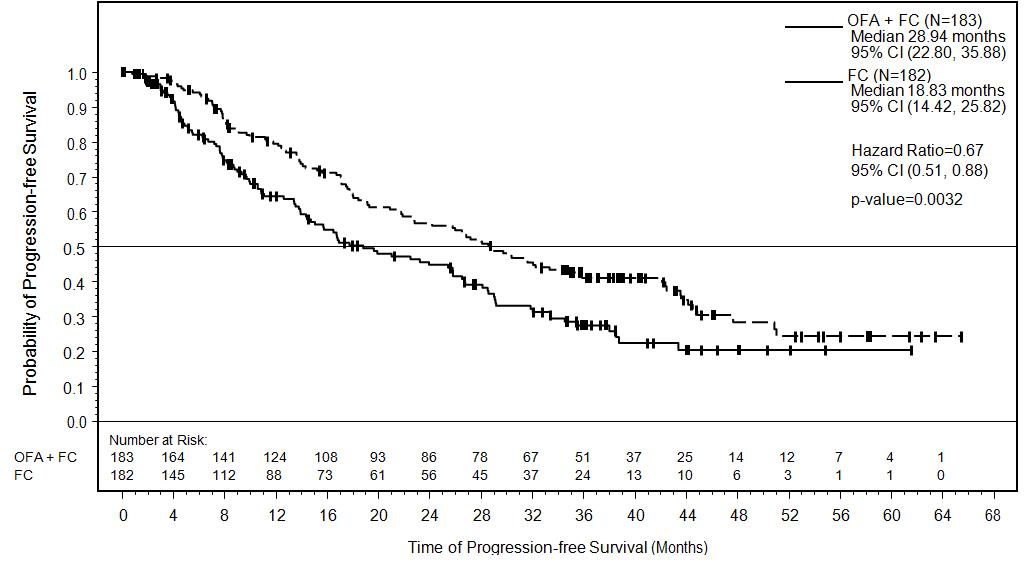
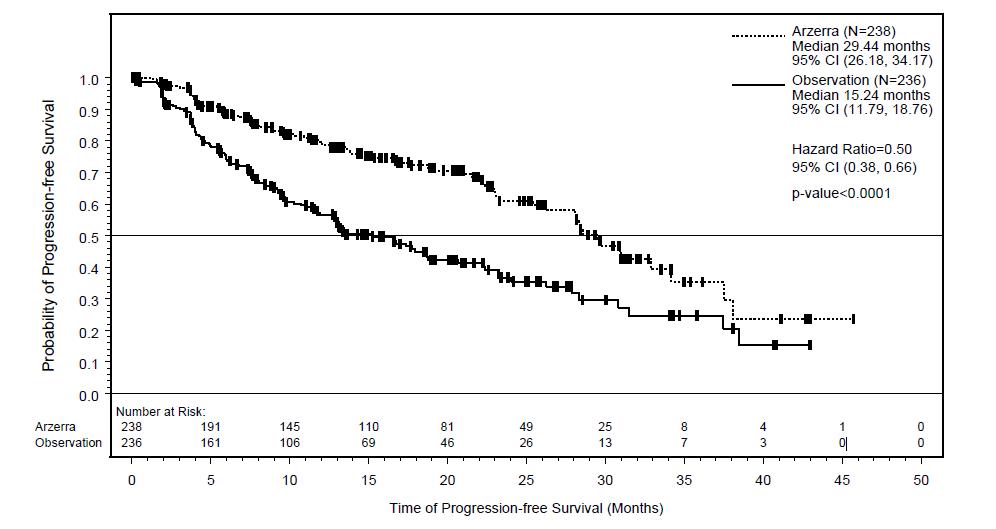
14 CLINICAL STUDIES14.1 Previously Untreated CLL - The efficacy of ARZERRA was evaluated in a randomized, open-label, parallel-arm study; 447 patients previously untreated for CLL were randomized to receive ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGARZERRA (ofatumumab) is a sterile, clear to opalescent, colorless, preservative-free liquid concentrate (20 mg/mL) for dilution and intravenous administration provided in single-use glass vials ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to contact a healthcare professional for any of the following: Signs and symptoms of infusion reactions including fever, chills, rash, or breathing problems within 24 hours of ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - NDC 0078-0669-13 - Arzerra® (ofatumumab) Injection, for Intravenous Infusion - 100 mg/5 mL(20mg/mL) Rx only - For Intravenous Infusion Only. Must Be Diluted Prior ...
-
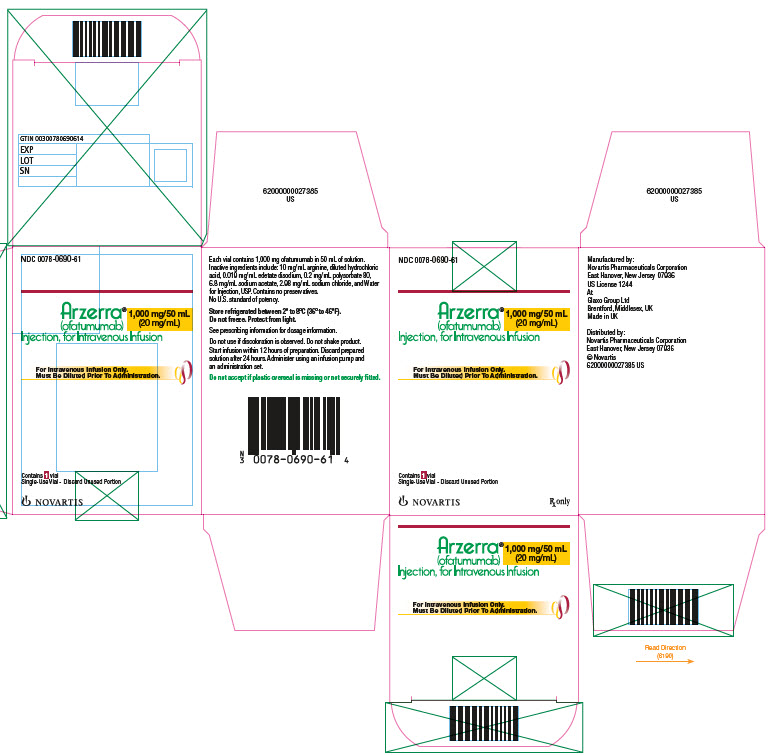
PRINCIPAL DISPLAY PANELPrincipal Display Panel - NDC 0078-0690-61 - Arzerra® (ofatumumab) Injection, for Intravenous Infusion - 1000 mg/50 mL(20mg/mL) Rx only - For Intravenous Infusion Only. Must Be Diluted ...
-
INGREDIENTS AND APPEARANCEProduct Information