Label: ARESTIN- minocycline hydrochloride powder
- NDC Code(s): 65976-100-01, 65976-100-12, 65976-100-24
- Packager: OraPharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
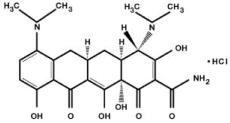
DESCRIPTION ARESTIN (minocycline hydrochloride) microspheres, 1 mg is a subgingival sustained-release product containing the antibiotic minocycline hydrochloride incorporated into a bioresorbable polymer ...
-
CLINICAL PHARMACOLOGYMechanism of Action - The mechanism of action of ARESTIN as an adjunct to scaling and root planing procedures for reduction of pocket depth in patients with adult periodontitis is ...
-
INDICATIONS AND USE ARESTIN is indicated as an adjunct to scaling and root planing procedures for reduction of pocket depth in patients with adult periodontitis. ARESTIN may be used as part of a periodontal ...
-
CONTRAINDICATIONS ARESTIN should not be used in any patient who has a known sensitivity to minocycline or tetracyclines.
-
WARNINGS THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY, AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH ...
-
PRECAUTIONSHypersensitivity Reactions and Hypersensitivity Syndrome - The following adverse events have been reported with minocycline products when taken orally. Hypersensitivity reactions and ...
-
ADVERSE REACTIONSThe most frequently reported non-dental, treatment-emergent adverse events in the 3 multicenter US trials were headache, infection, flu syndrome, and pain. Table 5: Adverse Events (AEs ...
-
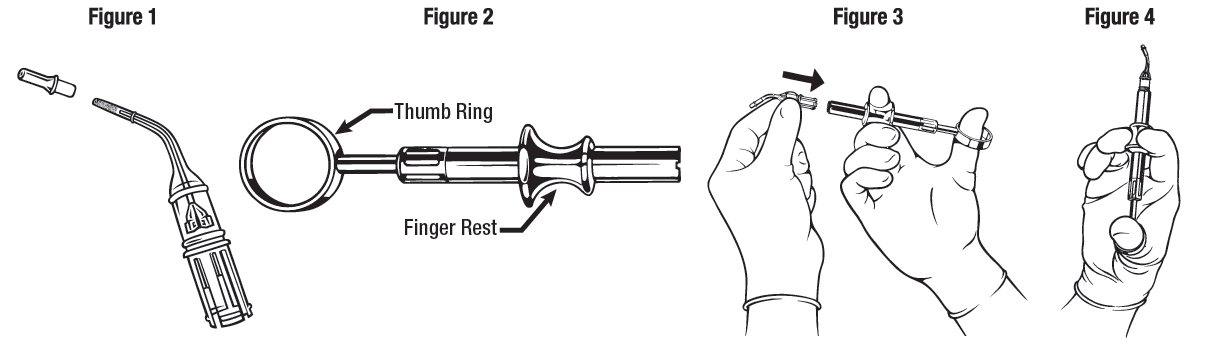
DOSAGE AND ADMINISTRATION ARESTIN is provided as a dry powder, packaged in a unit-dose cartridge with a deformable tip (see Figure 1), which is inserted into a spring-loaded cartridge handle mechanism (see Figure 2) to ...
-
HOW SUPPLIED ARESTIN® (minocycline hydrochloride) microspheres, 1 mg is supplied as follows: NDC 65976-100-01 1 unit-dose cartridge with desiccant in a heat-sealed, foil-laminated pouch - NDC 65976-100-24 ...
-
SPL UNCLASSIFIED SECTIONDistributed by: OraPharma, a division of Bausch Health US, LLC - Bridgewater, NJ 08807 USA - ARESTIN is a trademark of Bausch Health Companies Inc. or its affiliates. © 2024 Bausch Health Companies ...
-
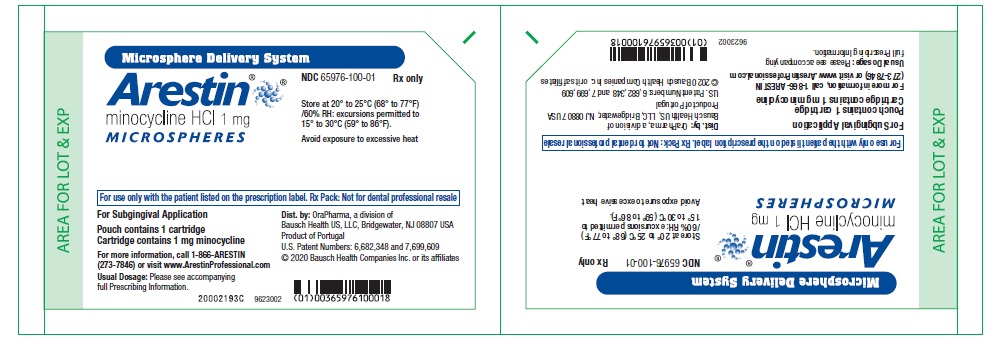
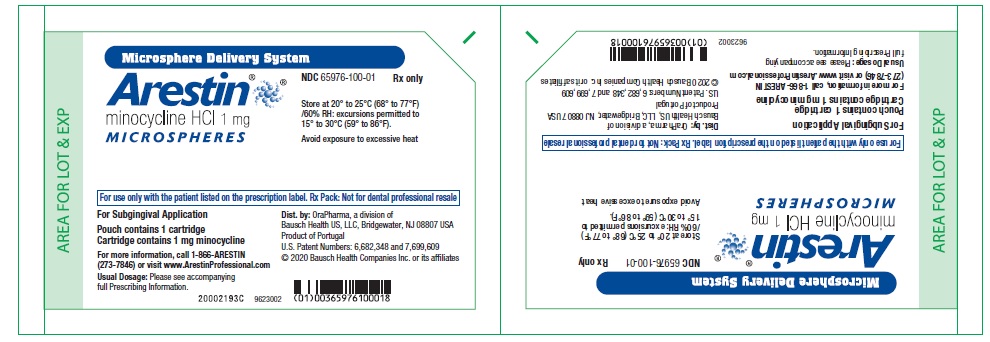
PRINCIPAL DISPLAY PANEL - 1 mg CartonNDC 65976-100-24 - Microsphere Delivery System - Arestin® minocycline HCl 1mg - MICROSPHERES - Store at 20° to 25°C (68° to 77°F) /60% RH: excursions permitted to - 15° to 30°C (59° to ...
-
Principal Display Panel – 1 mg pouch containing 1 cartridge Microsphere Delivery System - Arestin® minocycline HCl 1 mg - MICROSPHERES - For use only with the patient listed on the prescription label. Rx Pack: Not for dental professional resale - For ...
-
INGREDIENTS AND APPEARANCEProduct Information