Label: ARAZLO- tazarotene lotion
- NDC Code(s): 0187-2098-03, 0187-2098-10, 0187-2098-45
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARAZLO safely and effectively. See full prescribing information for ARAZLO. ARAZLO - ®(tazarotene) lotion, for topical use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEARAZLO - ®(tazarotene) lotion, 0.045% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of ARAZLO to the affected areas once daily. Avoid the eyes, mouth, paranasal creases, and mucous membranes. If ARAZLO gets in or near eyes, rinse thoroughly with water. ARAZLO ...
-
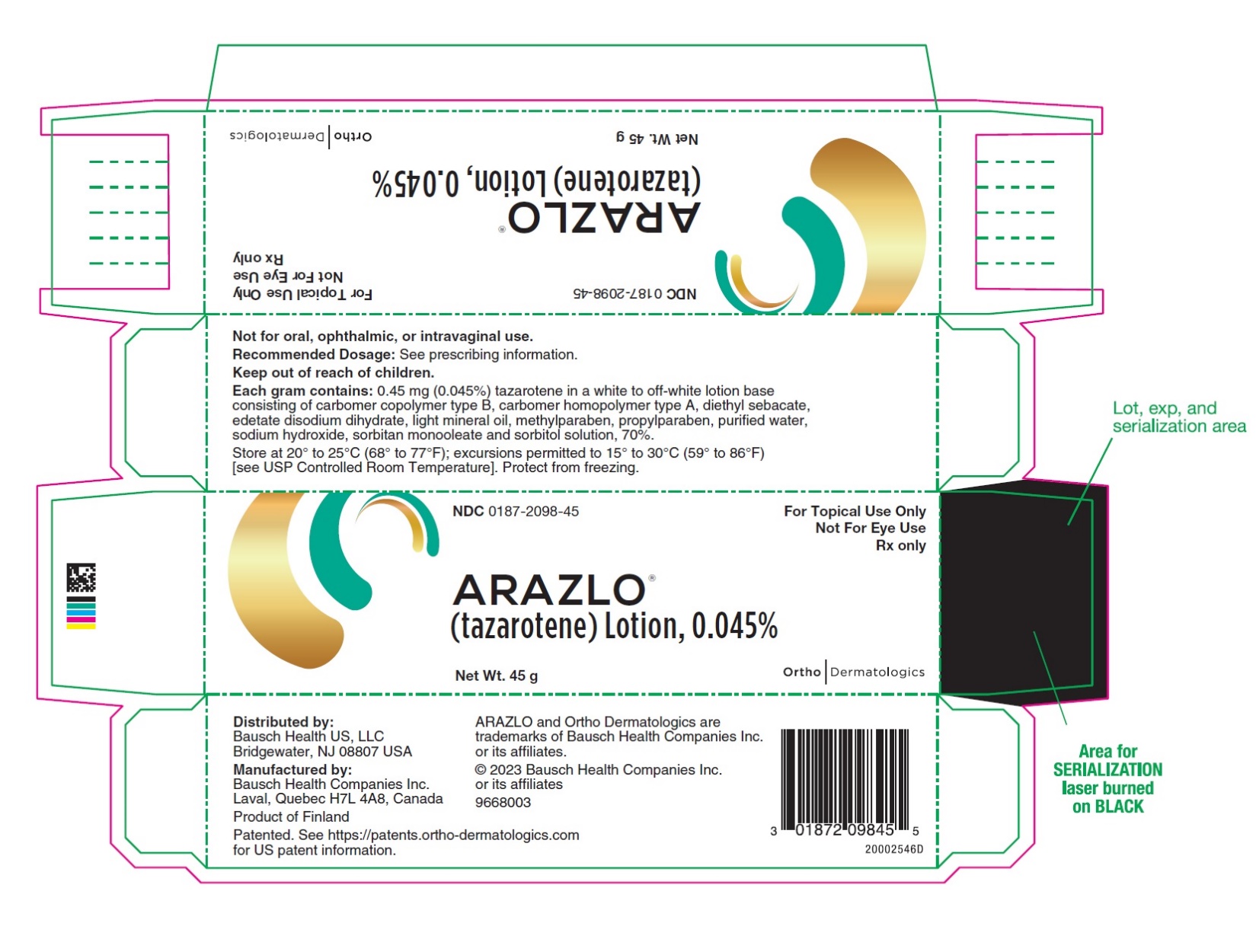
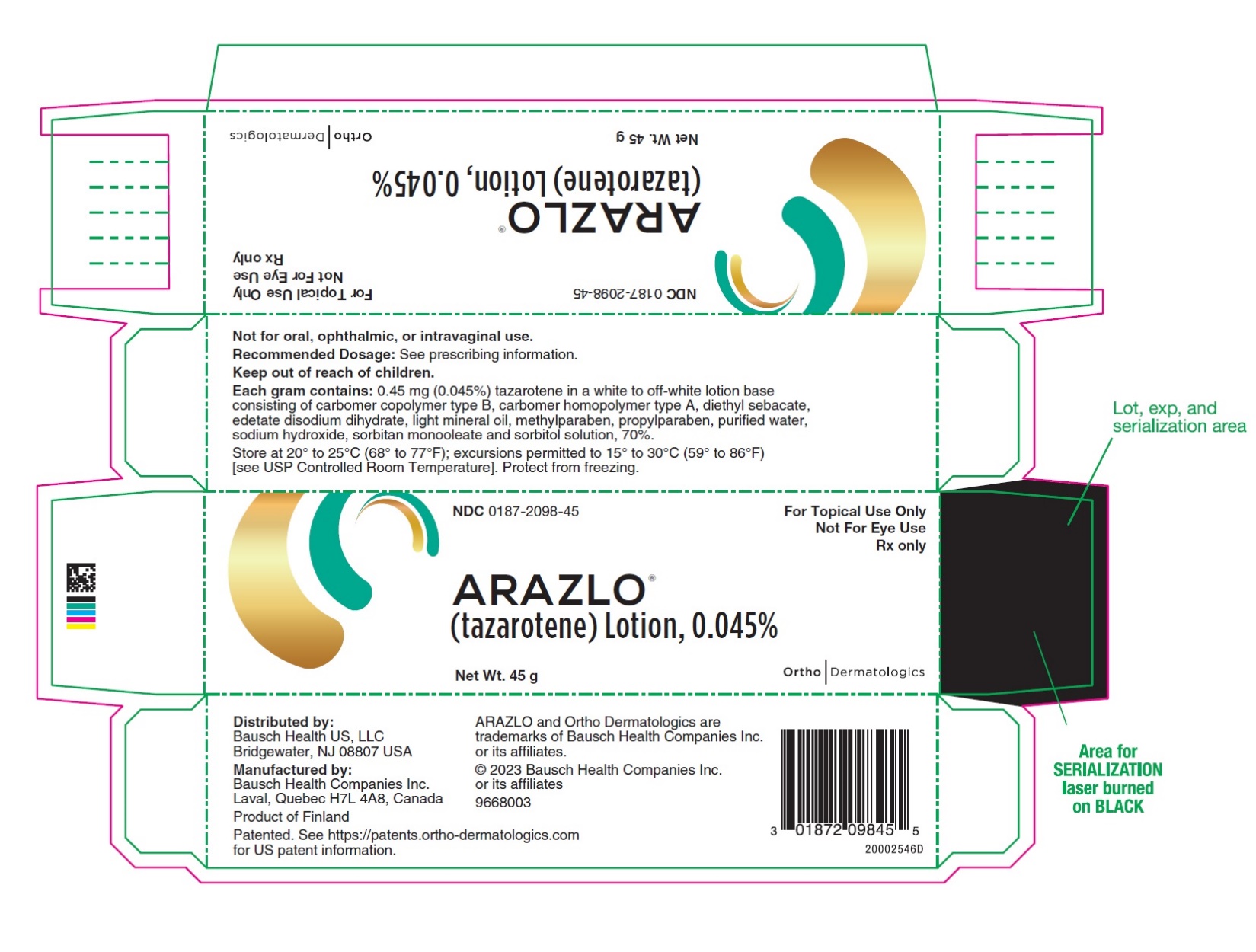
3 DOSAGE FORMS AND STRENGTHSLotion, 0.045% Each gram of ARAZLO contains 0.45 mg (0.045%) tazarotene in a white to off-white topical lotion.
-
4 CONTRAINDICATIONSARAZLO is contraindicated in pregnancy. ARAZLO may cause fetal harm when administered to a pregnant patient - [see - Warnings and Precautions (5.1), Use in Specific Populations (8.1 ...
-
5 WARNINGS AND PRECAUTIONS5.1 Embryofetal Toxicity - Based on data from animal reproduction studies, retinoid pharmacology and the potential for systemic absorption, ARAZLO may cause fetal harm when administered to a ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more detail in other sections of the labeling: Embryofetal toxicity - [see - Warnings and Precautions (5.1) ...
-
7 DRUG INTERACTIONSNo formal drug-drug interaction studies were conducted with ARAZLO. Concomitant use with oxidizing agents, as benzoyl peroxide, may cause degradation of tazarotene and may reduce the clinical ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - ARAZLO is contraindicated in pregnancy. There are no available data on ARAZLO use in pregnant patients to inform a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEOral ingestion of the drug may lead to the same adverse effects as those associated with excessive oral intake of Vitamin A (hypervitaminosis A) or other retinoids. If oral ingestion occurs ...
-
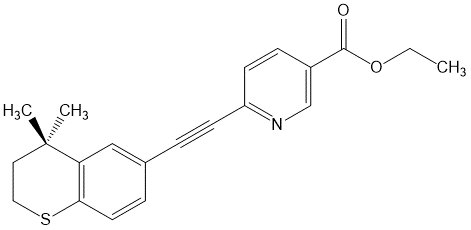
11 DESCRIPTIONARAZLO (tazarotene) is a white to off-white lotion containing 0.045% tazarotene by weight for topical administration. Tazarotene is a member of the acetylenic class of retinoids. The chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tazarotene is a retinoid prodrug which is converted to its active form, tazarotenic acid, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of ...
-
14 CLINICAL STUDIESThe safety and efficacy of once daily use of ARAZLO for the treatment of acne vulgaris were assessed in two multicenter, randomized, double-blind clinical trials in subjects 9 years and older with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGARAZLO (tazarotene) Lotion, 0.045% is a white to off-white lotion supplied in a white aluminum tube as follows: 45 g (NDC 0187-2098-45) Storage and Handling Conditions - Store at 20° to 25°C ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Embryofetal Toxicity - Inform patients of childbearing potential of the potential risk to a fetus. To avoid ...
-
Patient InformationPATIENT INFORMATION - ARAZLO - ®(ah-RAZ-low) (tazarotene) lotion, for topical use - Important information:ARAZLO is for use on skin only. Do not use ARAZLO ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 45 gram CartonNDC0187-2098-45 - For Topical Use Only - Not For Eye Use - Rx only - ARAZLO® (tazarotene) Lotion, 0.045% Net Wt. 45 g - OrthoDermatologics
-
INGREDIENTS AND APPEARANCEProduct Information