Label: ANZEMET- dolasetron mesylate tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 30698-220-10 - Packager: Validus Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONANZEMET ® Tablets - (dolasetron mesylate, USP) Rx Only
-
DESCRIPTION
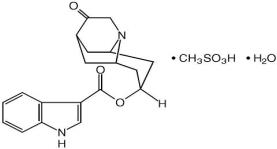
ANZEMET (dolasetron mesylate, USP) is an antinauseant and antiemetic agent. Chemically, dolasetron mesylate is (2α,6α,8α,9aß)-octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl-1H-indole-3-carboxylate ...
-
CLINICAL PHARMACOLOGY
Dolasetron mesylate and its active metabolite, hydrodolasetron (MDL 74,156), are selective serotonin 5-HT3 receptor antagonists not shown to have activity at other known serotonin receptors and ...
-
INDICATIONS AND USAGE
ANZEMET Tablets are indicated for the prevention of nausea and vomiting associated with moderately emetogenic cancer chemotherapy, including initial and repeat courses in adults and children 2 ...
-
CONTRAINDICATIONS
ANZEMET Tablets are contraindicated in patients known to have hypersensitivity to the drug.
-
WARNINGS
QT Interval Prolongation - ANZEMET prolongs the QT interval in a dose dependent fashion. Torsade de Pointes has been reported during post-marketing experience. Avoid ANZEMET in patients with ...
-
PRECAUTIONS
General - Dolasetron should be administered with caution in patients who have or may develop prolongation of cardiac conduction intervals, particularly QTc. These include patients with ...
-
ADVERSE REACTIONS
In controlled clinical trials, 943 adult cancer patients received ANZEMET Tablets. These patients were receiving concurrent chemotherapy, predominantly cyclophosphamide and doxorubicin regimens ...
-
OVERDOSAGE
There is no known specific antidote for dolasetron mesylate, and patients with suspected overdose should be managed with supportive therapy. Individual doses as large as 5 mg/kg intravenously or ...
-
DOSAGE AND ADMINISTRATION
The recommended doses of ANZEMET Tablets should not be exceeded. Adults - The recommended oral dosage of ANZEMET (dolasetron mesylate) is 100 mg given within one hour before chemotherapy. Pediatric ...
-
HOW SUPPLIED
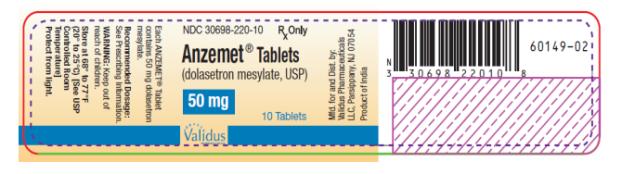
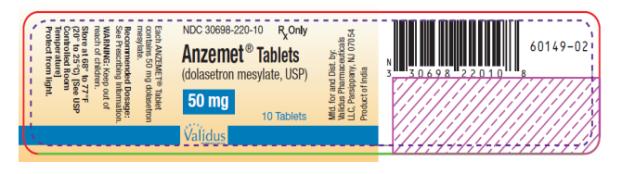
ANZEMET® Tablets - (dolasetron mesylate, USP) StrengthQuantityNDC NumberDescription - 50 mg10 ct Bottle30698-220-10Light pink, film coated, round tablet debossed with “A” on one side ...
-
PATIENT COUNSELING INFORMATION
Patients should be informed that ANZEMET may cause serious cardiac arrhythmias such as QT prolongation or heart block. Patients should be instructed to tell their health care provider right away ...
-
PRINCIPAL DISPLAY PANEL
NDC 30698-220-10 - Anzemet® Tablets - (dolasetron mesylate, USP) 50 mg - 10 Tablets - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information