Label: ANORO ELLIPTA- umeclidinium bromide and vilanterol trifenatate powder
- NDC Code(s): 0173-0869-06, 0173-0869-10, 0173-0869-61
- Packager: GlaxoSmithKline LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ANORO ELLIPTA safely and effectively. See full prescribing information for ANORO ELLIPTA. ANORO ELLIPTA (umeclidinium and ...These highlights do not include all the information needed to use ANORO ELLIPTA safely and effectively. See full prescribing information for ANORO ELLIPTA.
ANORO ELLIPTA (umeclidinium and vilanterol inhalation powder), for oral inhalation use
Initial U.S. Approval: 2013INDICATIONS AND USAGE
ANORO ELLIPTA is a combination of umeclidinium, an anticholinergic, and vilanterol, a long-acting beta2-adrenergic agonist (LABA), indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). (1)
Limitations of Use: Not indicated for relief of acute bronchospasm or for the treatment of asthma. (1, 5.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Inhalation powder: 62.5 mcg umeclidinium and 25 mcg vilanterol (62.5/25 mcg) per actuation. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- LABA monotherapy (without an inhaled corticosteroid) for asthma increases the risk of serious asthma-related events. (5.1)
- •
- Do not initiate in acutely deteriorating COPD. Do not use to treat acute symptoms. (5.2)
- •
- Do not use in combination with additional therapy containing a LABA because of risk of overdose. (5.3)
- •
- If paradoxical bronchospasm occurs, discontinue ANORO ELLIPTA and institute alternative therapy. (5.5)
- •
- Use with caution in patients with cardiovascular disorders because of beta-adrenergic stimulation. (5.7)
- •
- Use with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, and ketoacidosis. (5.8)
- •
- Worsening of narrow-angle glaucoma may occur. Use with caution in patients with narrow-angle glaucoma and instruct patients to contact a healthcare provider immediately if symptoms occur. (5.9)
- •
- Worsening of urinary retention may occur. Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur. (5.10)
- •
- Be alert to hypokalemia and hyperglycemia. (5.11)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥1% and more common than placebo) are pharyngitis, sinusitis, lower respiratory tract infection, constipation, diarrhea, pain in extremity, muscle spasms, neck pain, and chest pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Strong cytochrome P450 3A4 inhibitors (e.g., ketoconazole): Use with caution. May cause cardiovascular effects. (7.1)
- •
- Monoamine oxidase inhibitors and tricyclic antidepressants: Use with extreme caution. May potentiate effect of vilanterol on cardiovascular system. (7.2)
- •
- Beta-blockers: Use with caution. May block bronchodilatory effects of beta-agonists and produce severe bronchospasm. (7.3)
- •
- Diuretics: Use with caution. Electrocardiographic changes and/or hypokalemia associated with non–potassium-sparing diuretics may worsen with concomitant beta-agonists. (7.4)
- •
- Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of ANORO ELLIPTA with other anticholinergic-containing drugs. (7.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
5.2 Deterioration of Disease and Acute Episodes
5.3 Avoid Excessive Use of ANORO ELLIPTA and Avoid Use with Other Long-acting Beta2-agonists
5.4 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
5.5 Paradoxical Bronchospasm
5.6 Hypersensitivity Reactions, including Anaphylaxis
5.7 Cardiovascular Effects
5.8 Coexisting Conditions
5.9 Worsening of Narrow-Angle Glaucoma
5.10 Worsening of Urinary Retention
5.11 Hypokalemia and Hyperglycemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
7.2 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, and QTc Prolonging Drugs
7.3 Beta-adrenergic Receptor Blocking Agents
7.4 Non–Potassium-Sparing Diuretics
7.5 Anticholinergics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Dose-Ranging Trials
14.2 Confirmatory Trials
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGEANORO ELLIPTA is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Limitations of Use - ANORO ELLIPTA is NOT indicated for the relief of acute ...
ANORO ELLIPTA is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
Limitations of Use
ANORO ELLIPTA is NOT indicated for the relief of acute bronchospasm or for the treatment of asthma. The safety and effectiveness of ANORO ELLIPTA in asthma have not been established.
Close -
2 DOSAGE AND ADMINISTRATIONThe recommended dosage of ANORO ELLIPTA for maintenance treatment of COPD is 62.5 mcg umeclidinium and 25 mcg vilanterol (1 actuation of ANORO ELLIPTA 62.5/25 mcg) once daily by oral ...
The recommended dosage of ANORO ELLIPTA for maintenance treatment of COPD is 62.5 mcg umeclidinium and 25 mcg vilanterol (1 actuation of ANORO ELLIPTA 62.5/25 mcg) once daily by oral inhalation.
- •
- ANORO ELLIPTA should be used at the same time every day. Do not use ANORO ELLIPTA more than 1 time every 24 hours.
- •
- No dosage adjustment is required for geriatric patients, patients with renal impairment, or patients with moderate hepatic impairment [see Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHSInhalation powder: 62.5 mcg umeclidinium and 25 mcg vilanterol (62.5/25 mcg) per actuation.
-
4 CONTRAINDICATIONSANORO ELLIPTA is contraindicated in: • patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to umeclidinium, vilanterol, or any of the excipients [see ...
ANORO ELLIPTA is contraindicated in:
- •
- patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to umeclidinium, vilanterol, or any of the excipients [see Warnings and Precautions (5.6), Description (11)].
- •
- use of a long-acting beta2-adrenergic agonist (LABA), including vilanterol, one of the active ingredients in ANORO ELLIPTA, without an inhaled corticosteroid (ICS), in patients with asthma [see Warnings and Precautions (5.1)]. ANORO ELLIPTA is not indicated for the treatment of asthma.
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death - The safety and effectiveness of ANORO ELLIPTA in patients with asthma have not been established. ANORO ELLIPTA is not ...
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
The safety and effectiveness of ANORO ELLIPTA in patients with asthma have not been established. ANORO ELLIPTA is not indicated for the treatment of asthma [see Contraindications (4)].
Use of LABA as monotherapy (without ICS) for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
Salmeterol Multicenter Asthma Research Trial (SMART)
A 28-week, placebo-controlled, US trial that compared the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol (13/13,176 in subjects treated with salmeterol vs. 3/13,179 in subjects treated with placebo; relative risk: 4.37 [95% CI: 1.25, 15.34]). The increased risk of asthma-related death is considered a class effect of LABAs, including vilanterol, one of the active ingredients in ANORO ELLIPTA.
No trial adequate to determine whether the rate of asthma-related death is increased in subjects treated with ANORO ELLIPTA has been conducted.
Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
5.2 Deterioration of Disease and Acute Episodes
ANORO ELLIPTA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD. ANORO ELLIPTA has not been studied in subjects with acutely deteriorating COPD. The initiation of ANORO ELLIPTA in this setting is not appropriate.
If ANORO ELLIPTA no longer controls symptoms of bronchoconstriction; the patient’s inhaled, short-acting beta2-agonist becomes less effective; or the patient needs more short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, re-evaluate the patient and the COPD treatment regimen at once. The daily dose of ANORO ELLIPTA should not be increased. Increasing use of inhaled, short-acting beta2-agonists is a signal of deteriorating disease. In this situation, the patient requires immediate re-evaluation with reassessment of the treatment regimen, giving special consideration to the need for additional therapeutic options. Patients should not use more than 1 inhalation of ANORO ELLIPTA once daily.
ANORO ELLIPTA should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. ANORO ELLIPTA has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist.
When beginning treatment with ANORO ELLIPTA, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs and to use them only for symptomatic relief of acute respiratory symptoms. When prescribing ANORO ELLIPTA, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient on how it should be used.
5.3 Avoid Excessive Use of ANORO ELLIPTA and Avoid Use with Other Long-acting Beta2-agonists
ANORO ELLIPTA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other therapies containing LABAs, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using ANORO ELLIPTA should not use another therapy containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
5.4 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the coadministration of ANORO ELLIPTA with ketoconazole and other known strong cytochrome P450 3A4 (CYP3A4) inhibitors (including, but not limited to, ritonavir, clarithromycin, conivaptan, indinavir, itraconazole, lopinavir, nefazodone, nelfinavir, saquinavir, telithromycin, troleandomycin, voriconazole) because increased cardiovascular adverse effects may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
5.5 Paradoxical Bronchospasm
As with other inhaled therapies, ANORO ELLIPTA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with ANORO ELLIPTA, it should be treated immediately with an inhaled, short-acting bronchodilator; ANORO ELLIPTA should be discontinued immediately; and alternative therapy should be instituted.
5.6 Hypersensitivity Reactions, including Anaphylaxis
Hypersensitivity reactions such as anaphylaxis, angioedema, rash, and urticaria may occur after administration of ANORO ELLIPTA. Discontinue ANORO ELLIPTA if such reactions occur. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of other powder medications containing lactose; therefore, patients with severe milk protein allergy should not use ANORO ELLIPTA [see Contraindications (4), Adverse Reactions (6.2)].
5.7 Cardiovascular Effects
Vilanterol, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles. If such effects occur, ANORO ELLIPTA may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiographic changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown [see Clinical Pharmacology (12.2)]. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
ANORO ELLIPTA, like other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
In a 52-week trial of subjects with COPD, the exposure-adjusted rates for any on-treatment major adverse cardiac event, including non-fatal central nervous system hemorrhages and cerebrovascular conditions, non-fatal myocardial infarction, non-fatal acute myocardial infarction, and adjudicated on-treatment death due to cardiovascular events, was 2.2 per 100 patient-years for fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 mcg (n = 4,151), 1.9 per 100 patient-years for fluticasone furoate/vilanterol 100/25 mcg (n = 4,134), and 2.2 per 100 patient-years for ANORO ELLIPTA (n = 2,070). Adjudicated on‑treatment deaths due to cardiovascular events occurred in 20 of 4,151 patients (0.54 per 100 patient-years) receiving fluticasone furoate/umeclidinium/vilanterol, 27 of 4,134 patients (0.78 per 100 patient-years) receiving fluticasone furoate/vilanterol, and 16 of 2,070 patients (0.94 per 100 patient-years) receiving ANORO ELLIPTA.
5.8 Coexisting Conditions
ANORO ELLIPTA, like all therapies containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2-adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.9 Worsening of Narrow-Angle Glaucoma
ANORO ELLIPTA should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should also be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos, or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develop.
5.10 Worsening of Urinary Retention
ANORO ELLIPTA, like all therapies containing an anticholinergic, should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a healthcare provider immediately if any of these signs or symptoms develop.
Close5.11 Hypokalemia and Hyperglycemia
Beta-adrenergic agonist therapies may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. Beta-agonist therapies may produce transient hyperglycemia in some patients. In 4 clinical trials of 6-month duration evaluating ANORO ELLIPTA in subjects with COPD, there was no evidence of a treatment effect on serum glucose or potassium.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: • Serious asthma-related events–hospitalizations, intubations, death [see Warnings and Precautions ...
The following clinically significant adverse reactions are described elsewhere in labeling:
- •
- Serious asthma-related events–hospitalizations, intubations, death [see Warnings and Precautions (5.1)]
- •
- Paradoxical bronchospasm [see Warnings and Precautions (5.5)]
- •
- Cardiovascular effects [see Warnings and Precautions (5.7)]
- •
- Worsening of narrow-angle glaucoma [see Warnings and Precautions (5.9)]
- •
- Worsening of urinary retention [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical program for ANORO ELLIPTA included 8,138 subjects with COPD in four 6‑month lung function trials, one 12-month long-term safety study, and 9 other trials of shorter duration. A total of 1,124 subjects have received at least 1 dose of ANORO ELLIPTA (umeclidinium/vilanterol 62.5/25 mcg), and 1,330 subjects have received a higher dose of umeclidinium/vilanterol (125/25 mcg). The safety data described below are based on the four 6-month and one 12-month trials. Adverse reactions observed in the other trials were similar to those observed in the confirmatory trials.
6-Month Trials
The incidence of adverse reactions associated with ANORO ELLIPTA in Table 1 is based on four 6-month trials: 2 placebo-controlled trials (Trial 1 and Trial 2); N = 1,532 and N = 1,489, respectively) and 2 active-controlled trials (Trial 3 and Trial 4); N = 843 and N = 869, respectively). Of the 4,733 subjects, 68% were male and 84% were white. They had a mean age of 63 years and an average smoking history of 45 pack-years, with 50% identified as current smokers. At screening, the mean postbronchodilator percent predicted forced expiratory volume in 1 second (FEV1) was 48% (range: 13% to 76%), the mean postbronchodilator FEV1/forced vital capacity (FVC) ratio was 0.47 (range: 0.13 to 0.78), and the mean percent reversibility was 14% (range: -45% to 109%).
Subjects received 1 dose once daily of the following: ANORO ELLIPTA, umeclidinium/vilanterol 125/25 mcg, umeclidinium 62.5 mcg, umeclidinium 125 mcg, vilanterol 25 mcg, active control, or placebo.
Table 1. Adverse Reactions with ANORO ELLIPTA with ≥1% Incidence and More Common than Placebo in Subjects with Chronic Obstructive Pulmonary Disease Adverse Reaction
ANORO ELLIPTA
(n = 842)
%
Umeclidinium
62.5 mcg
(n = 418)
%
Vilanterol
25 mcg
(n = 1,034)
%
Placebo
(n = 555)
%
Infections and infestations
Pharyngitis
2
1
2
<1
Sinusitis
1
<1
1
<1
Lower respiratory tract infection
1
<1
<1
<1
Gastrointestinal disorders
Constipation
1
<1
<1
<1
Diarrhea
2
<1
2
1
Musculoskeletal and connective tissue disorders
Pain in extremity
2
<1
2
1
Muscle spasms
1
<1
<1
<1
Neck pain
1
<1
<1
<1
General disorders and administration site conditions
Chest pain
1
<1
<1
<1
Other adverse reactions with ANORO ELLIPTA observed with an incidence <1% but more common than placebo included the following: productive cough, dry mouth, dyspepsia, abdominal pain, gastroesophageal reflux disease, vomiting, musculoskeletal chest pain, chest discomfort, asthenia, atrial fibrillation, ventricular extrasystoles, supraventricular extrasystoles, myocardial infarction, pruritus, rash, and conjunctivitis.
12-Month Trial
In a long-term safety trial (Trial 5), 335 subjects were treated for up to 12 months with umeclidinium/vilanterol 125/25 mcg or placebo. The demographic and baseline characteristics of the long-term safety trial were similar to those of the placebo-controlled efficacy trials described above. Adverse reactions observed with a frequency of ≥1% in the group receiving umeclidinium/vilanterol 125/25 mcg that exceeded that in placebo in this trial were: headache, back pain, sinusitis, cough, urinary tract infection, arthralgia, nausea, vertigo, abdominal pain, pleuritic pain, viral respiratory tract infection, toothache, and diabetes mellitus.
Close6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postapproval use of ANORO ELLIPTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to ANORO ELLIPTA or a combination of these factors.
Cardiac Disorders
Palpitations.
Eye Disorders
Blurred vision, eye pain, glaucoma, increased intraocular pressure.
Immune System Disorders
Hypersensitivity reactions, including anaphylaxis, angioedema, and urticaria.
Nervous System Disorders
Dysgeusia, tremor.
Psychiatric Disorders
Anxiety.
Renal and Urinary Disorders
Dysuria, urinary retention.
Respiratory, Thoracic, and Mediastinal Disorders
Dysphonia, paradoxical bronchospasm.
-
7 DRUG INTERACTIONS7.1 Inhibitors of Cytochrome P450 3A4 - Vilanterol is a substrate of CYP3A4. Concomitant administration of the strong CYP3A4 inhibitor ketoconazole increases the systemic exposure to vilanterol ...
7.1 Inhibitors of Cytochrome P450 3A4
Vilanterol is a substrate of CYP3A4. Concomitant administration of the strong CYP3A4 inhibitor ketoconazole increases the systemic exposure to vilanterol. Caution should be exercised when considering the coadministration of ANORO ELLIPTA with ketoconazole and other known strong CYP3A4 inhibitors [see Warnings and Precautions (5.4), Clinical Pharmacology (12.3)].
7.2 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, and QTc Prolonging Drugs
Vilanterol, like other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval or within 2 weeks of discontinuation of such agents, because the effect of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval have an increased risk of ventricular arrhythmias.
7.3 Beta-adrenergic Receptor Blocking Agents
Beta-blockers not only block the pulmonary effect of beta-agonists, such as vilanterol, but may also produce severe bronchospasm in patients with COPD. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents for these patients; cardioselective beta-blockers could be considered, although they should be administered with caution.
7.4 Non–Potassium-Sparing Diuretics
The electrocardiographic changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with non–potassium-sparing diuretics.
Close7.5 Anticholinergics
There is potential for an additive interaction with concomitantly used anticholinergic medicines. Therefore, avoid coadministration of ANORO ELLIPTA with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.9, 5.10)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient data on the use of ANORO ELLIPTA or its individual components, umeclidinium and vilanterol, in pregnant women to inform a drug-associated ...
8.1 Pregnancy
Risk Summary
There are insufficient data on the use of ANORO ELLIPTA or its individual components, umeclidinium and vilanterol, in pregnant women to inform a drug-associated risk. (See Clinical Considerations.) In animal reproduction studies, umeclidinium administered via inhalation or subcutaneously to pregnant rats and rabbits was not associated with adverse effects on embryofetal development at exposures approximately 50 and 200 times, respectively, the human exposure at the maximum recommended human daily inhaled dose (MRHDID). Vilanterol administered via inhalation to pregnant rats and rabbits produced no fetal structural abnormalities at exposures approximately 70 times the MRHDID. (See Data.)
The estimated risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor or Delivery: ANORO ELLIPTA should be used during late gestation and labor only if the potential benefit justifies the potential for risks related to beta-agonists interfering with uterine contractility.
Data
Animal Data: The combination of umeclidinium and vilanterol has not been studied in pregnant animals. Studies in pregnant animals have been conducted with umeclidinium and vilanterol individually.
Umeclidinium: In separate embryofetal developmental studies, pregnant rats and rabbits received umeclidinium during the period of organogenesis at doses up to approximately 50 and 200 times the MRHDID, respectively (on an AUC basis at maternal inhalation doses up to 278 mcg/kg/day in rats and at maternal subcutaneous doses up to 180 mcg/kg/day in rabbits). No evidence of teratogenic effects was observed in either species.
In a perinatal and postnatal developmental study in rats, dams received umeclidinium during late gestation and lactation periods with no evidence of effects on offspring development at doses up to approximately 26 times the MRHDID (on an AUC basis at maternal subcutaneous doses up to 60 mcg/kg/day).
Vilanterol: In separate embryofetal developmental studies, pregnant rats and rabbits received vilanterol during the period of organogenesis at doses up to approximately 13,000 and 450 times, respectively, the MRHDID (on a mcg/m2 basis at maternal inhalation doses up to 33,700 mcg/kg/day in rats and on an AUC basis at maternal inhaled doses up to 5,740 mcg/kg/day in rabbits). No evidence of structural abnormalities was observed at any dose in rats or in rabbits up to approximately 70 times the MRHDID (on an AUC basis at maternal doses up to 591 mcg/kg/day in rabbits). However, fetal skeletal variations were observed in rabbits at approximately 450 times the MRHDID (on an AUC basis at maternal inhaled or subcutaneous doses of 5,740 or 300 mcg/kg/day, respectively). The skeletal variations included decreased or absent ossification in cervical vertebral centrum and metacarpals.
In a perinatal and postnatal developmental study in rats, dams received vilanterol during late gestation and the lactation periods at doses up to approximately 3,900 times the MRHDID (on a mcg/m2 basis at maternal oral doses up to 10,000 mcg/kg/day). No evidence of effects in offspring development was observed.
8.2 Lactation
Risk Summary
There is no information available on the presence of umeclidinium or vilanterol in human milk, the effects on the breastfed child, or the effects on milk production. Umeclidinium was detected in the plasma of offspring of lactating rats treated with umeclidinium suggesting its presence in maternal milk. (See Data.) The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ANORO ELLIPTA and any potential adverse effects on the breastfed child from umeclidinium or vilanterol or from the underlying maternal condition.
Data
Subcutaneous administration of umeclidinium to lactating rats at greater than or equal to 60 mcg/kg/day resulted in a quantifiable level of umeclidinium in 2 of 54 pups, which may indicate transfer of umeclidinium in rat milk.
8.4 Pediatric Use
The safety and effectiveness of ANORO ELLIPTA have not been established in pediatric patients. ANORO ELLIPTA is not indicated for use in pediatric patients.
8.5 Geriatric Use
Based on available data, no adjustment of the dosage of ANORO ELLIPTA in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
Clinical trials of ANORO ELLIPTA for COPD included 2,143 subjects aged 65 years and older and 478 subjects aged 75 years and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects.
8.6 Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh score of 7-9) showed no relevant increases in Cmax or AUC, nor did protein binding differ between subjects with moderate hepatic impairment and their healthy controls. Studies in subjects with severe hepatic impairment have not been performed [see Clinical Pharmacology (12.3)].
Close8.7 Renal Impairment
There were no significant increases in either umeclidinium or vilanterol exposure in subjects with severe renal impairment (CrCl <30 mL/min) compared with healthy subjects. No dosage adjustment is required in patients with renal impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEANORO ELLIPTA contains both umeclidinium and vilanterol; therefore, the risks associated with overdosage for the individual components described below apply to ANORO ELLIPTA. Treatment of ...
ANORO ELLIPTA contains both umeclidinium and vilanterol; therefore, the risks associated with overdosage for the individual components described below apply to ANORO ELLIPTA. Treatment of overdosage consists of discontinuation of ANORO ELLIPTA together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medicine can produce bronchospasm. Cardiac monitoring is recommended in cases of overdosage.
Umeclidinium
High doses of umeclidinium may lead to anticholinergic signs and symptoms.
Vilanterol
The expected signs and symptoms with overdosage of vilanterol are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms of beta-adrenergic stimulation (e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, hypokalemia, metabolic acidosis). As with all inhaled sympathomimetic medicines, cardiac arrest and even death may be associated with an overdose of vilanterol.
Close -
11 DESCRIPTIONANORO ELLIPTA is an inhalation powder drug product for delivery of a combination of umeclidinium (an anticholinergic) and vilanterol (a LABA) to patients by oral inhalation. Umeclidinium bromide ...
ANORO ELLIPTA is an inhalation powder drug product for delivery of a combination of umeclidinium (an anticholinergic) and vilanterol (a LABA) to patients by oral inhalation.
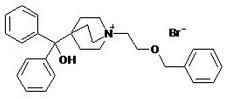
Umeclidinium bromide has the chemical name 1-[2-(benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-azoniabicyclo[2.2.2]octane bromide and the following chemical structure:
Umeclidinium bromide is a white powder with a molecular weight of 508.5, and the empirical formula is C29H34NO2•Br (as a quaternary ammonium bromide compound). It is slightly soluble in water.
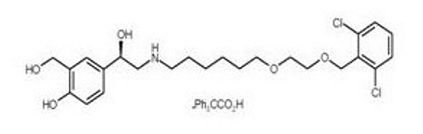
Vilanterol trifenatate has the chemical name triphenylacetic acid-4-{(1R)-2-[(6-{2-[(2,6-dicholorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol (1:1) and the following chemical structure:
Vilanterol trifenatate is a white powder with a molecular weight of 774.8, and the empirical formula is C24H33Cl2NO5•C20H16O2. It is practically insoluble in water.
ANORO ELLIPTA is a light grey and red plastic inhaler containing 2 foil blister strips. Each blister on one strip contains a white powder blend of micronized umeclidinium bromide (74.2 mcg equivalent to 62.5 mcg of umeclidinium), magnesium stearate (75 mcg), and lactose monohydrate (to 12.5 mg), and each blister on the other strip contains a white powder blend of micronized vilanterol trifenatate (40 mcg equivalent to 25 mcg of vilanterol), magnesium stearate (125 mcg), and lactose monohydrate (to 12.5 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within both blisters is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, ANORO ELLIPTA delivers 55 mcg of umeclidinium and 22 mcg of vilanterol per dose when tested at a flow rate of 60 L/min for 4 seconds.
In adult subjects with obstructive lung disease and severely compromised lung function (COPD with FEV1/FVC <70% and FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 66.5 L/min (range: 43.5 to 81.0 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ANORO ELLIPTA - ANORO ELLIPTA contains both umeclidinium and vilanterol. The mechanisms of action described below for the individual components apply to ANORO ELLIPTA ...
12.1 Mechanism of Action
ANORO ELLIPTA
ANORO ELLIPTA contains both umeclidinium and vilanterol. The mechanisms of action described below for the individual components apply to ANORO ELLIPTA. These drugs represent 2 different classes of medications (an anticholinergic and a LABA) each having different effects on clinical and physiological indices.
Umeclidinium
Umeclidinium is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine- and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of umeclidinium is predominantly a site-specific effect.
Vilanterol
Vilanterol is a LABA. In vitro tests have shown the functional selectivity of vilanterol was similar to salmeterol. The clinical relevance of this in vitro finding is unknown.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenergic agonist drugs, including vilanterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′,5′-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
12.2 Pharmacodynamics
Cardiac Electrophysiology
Healthy Subjects: QTc interval prolongation was studied in a double-blind, multiple-dose, placebo- and positive-controlled crossover study in 86 healthy subjects. The maximum mean (95% upper confidence bound) difference in QTcF from placebo after baseline correction was 4.6 (7.1) milliseconds and 8.2 (10.7) milliseconds for umeclidinium/vilanterol 125/25 mcg and umeclidinium/vilanterol 500/100 mcg (8/4 times the recommended dosage), respectively.
A dose-dependent increase in heart rate was also observed. The maximum mean (95% upper confidence bound) difference in heart rate from placebo after baseline correction was 8.8 (10.5) beats/min and 20.5 (22.3) beats/min seen 10 minutes after dosing for umeclidinium/vilanterol 125/25 mcg and umeclidinium/vilanterol 500/100 mcg, respectively.
Subjects with Chronic Obstructive Pulmonary Disease: The effect of ANORO ELLIPTA on cardiac rhythm in subjects diagnosed with COPD was assessed using 24-hour Holter monitoring in 6- and 12-month trials: 53 subjects received ANORO ELLIPTA, 281 subjects received umeclidinium/vilanterol 125/25 mcg, and 182 subjects received placebo. No clinically meaningful effects on cardiac rhythm were observed.
Close12.3 Pharmacokinetics
Linear pharmacokinetics was observed for umeclidinium (62.5 to 500 mcg) and vilanterol (25 to 100 mcg).
Absorption
Umeclidinium: Umeclidinium plasma levels may not predict therapeutic effect. Following inhaled administration of umeclidinium in healthy subjects, Cmax occurred at 5 to 15 minutes. Umeclidinium is mostly absorbed from the lung after inhaled doses with minimum contribution from oral absorption. Following repeat dosing of inhaled ANORO ELLIPTA, steady state was achieved within 14 days with up to 1.8-fold accumulation.
Vilanterol: Vilanterol plasma levels may not predict therapeutic effect. Following inhaled administration of vilanterol in healthy subjects, Cmax occurred at 5 to 15 minutes. Vilanterol is mostly absorbed from the lung after inhaled doses with negligible contribution from oral absorption. Following repeat dosing of inhaled ANORO ELLIPTA, steady state was achieved within 14 days with up to 1.7-fold accumulation.
Distribution
Umeclidinium: Following intravenous administration to healthy subjects, the mean volume of distribution was 86 L. In vitro plasma protein binding in human plasma was on average 89%.
Vilanterol: Following intravenous administration to healthy subjects, the mean volume of distribution at steady state was 165 L. In vitro plasma protein binding in human plasma was on average 94%.
Elimination
Metabolism: Umeclidinium: In vitro data showed that umeclidinium is primarily metabolized by the enzyme cytochrome P450 2D6 (CYP2D6) and is a substrate for the P-glycoprotein (P-gp) transporter. The primary metabolic routes for umeclidinium are oxidative (hydroxylation, O-dealkylation) followed by conjugation (e.g., glucuronidation), resulting in a range of metabolites with either reduced pharmacological activity or for which the pharmacological activity has not been established. Systemic exposure to the metabolites is low.
Vilanterol: In vitro data showed that vilanterol is metabolized principally by CYP3A4 and is a substrate for the P-gp transporter. Vilanterol is metabolized to a range of metabolites with significantly reduced β1- and β2-agonist activity.
Excretion: Umeclidinium: The effective half-life after once-daily oral dosing is 11 hours. Following intravenous dosing with radiolabeled umeclidinium, mass balance showed 58% of the radiolabel in the feces and 22% in the urine. The excretion of the drug-related material in the feces following intravenous dosing indicated elimination in the bile. Following oral dosing to healthy male subjects, radiolabel recovered in feces was 92% of the total dose and that in urine was <1% of the total dose, suggesting negligible oral absorption.
Vilanterol: The effective half-life for vilanterol, as determined from inhalation administration of multiple doses, is 11 hours. Following oral administration of radiolabeled vilanterol, mass balance showed 70% of the radiolabel in the urine and 30% in the feces.
Specific Populations
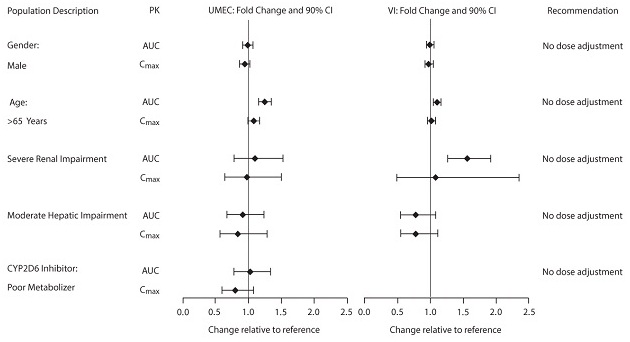
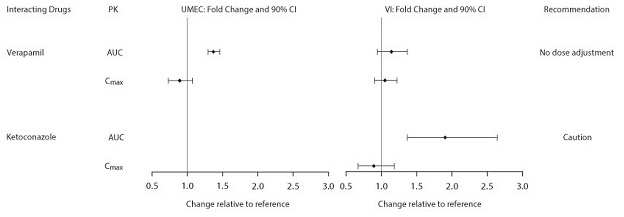
The effects of renal and hepatic impairment and other intrinsic factors on the pharmacokinetics of umeclidinium and vilanterol are shown in Figure 1. Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age (40 to 93 years) (Figure 1), gender (69% male) (Figure 1), inhaled corticosteroid use (48%), or weight (34 to 161 kg) on systemic exposure of either umeclidinium or vilanterol. In addition, there was no evidence of a clinically significant effect of race.
Figure 1. Impact of Intrinsic Factors on the Pharmacokinetics (PK) of Umeclidinium (UMEC) and Vilanterol (VI)
Patients with Hepatic Impairment: The impact of hepatic impairment on the pharmacokinetics of ANORO ELLIPTA has been evaluated in subjects with moderate hepatic impairment (Child-Pugh score of 7-9). There was no evidence of an increase in systemic exposure to either umeclidinium or vilanterol (Cmax and AUC) (Figure 1). There was no evidence of altered protein binding in subjects with moderate hepatic impairment compared with healthy subjects. ANORO ELLIPTA has not been evaluated in subjects with severe hepatic impairment.
Patients with Renal Impairment: The pharmacokinetics of ANORO ELLIPTA has been evaluated in subjects with severe renal impairment (CrCl <30 mL/min). Umeclidinium systemic exposure was not increased and vilanterol systemic exposure (AUC(0-24)) was 56% higher in subjects with severe renal impairment compared with healthy subjects (Figure 1). There was no evidence of altered protein binding in subjects with severe renal impairment compared with healthy subjects.
Drug Interaction Studies
When umeclidinium and vilanterol were administered in combination by the inhaled route, the pharmacokinetic parameters for each component were similar to those observed when each active substance was administered separately.
Inhibitors of Cytochrome P450 3A4: Vilanterol is a substrate of CYP3A4. A double-blind, repeat-dose, 2-way crossover drug interaction trial was conducted in healthy subjects to investigate the pharmacokinetic and pharmacodynamic effects of vilanterol 25 mcg as an inhalation powder with ketoconazole 400 mg. The plasma concentrations of vilanterol were higher after single and repeated doses when coadministered with ketoconazole than with placebo (Figure 2). The increase in vilanterol exposure was not associated with an increase in beta-agonist–related systemic effects on heart rate or blood potassium.
Inhibitors of Cytochrome P450 2D6: In vitro metabolism of umeclidinium is mediated primarily by CYP2D6. However, no clinically meaningful difference in systemic exposure to umeclidinium (500 mcg) (8 times the approved dose) was observed following repeat daily inhaled dosing in CYP2D6 normal (ultrarapid, extensive, and intermediate metabolizers) and poor metabolizer subjects (Figure 1).
Inhibitors of P-glycoprotein: Umeclidinium and vilanterol are both substrates of P-gp. The effect of the moderate P-gp transporter inhibitor verapamil (240 mg once daily) on the steady-state pharmacokinetics of umeclidinium and vilanterol was assessed in healthy subjects. No effect on umeclidinium or vilanterol Cmax was observed; however, an approximately 1.4-fold increase in umeclidinium AUC was observed with no effect on vilanterol AUC (Figure 2).
Figure 2. Impact of Extrinsic Factors on the Pharmacokinetics (PK) of Umeclidinium (UMEC) and Vilanterol (VI)
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - ANORO ELLIPTA - No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with ANORO ELLIPTA; however ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
ANORO ELLIPTA
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with ANORO ELLIPTA; however, studies are available for the individual components, umeclidinium and vilanterol, as described below.
Umeclidinium
Umeclidinium produced no treatment-related increases in the incidence of tumors in 2-year inhalation studies in rats and mice at inhaled doses up to 137 and 295/200 mcg/kg/day (male/female), respectively (approximately 20 and 25/20 times, respectively, the MRHDID for adults on an AUC basis).
Umeclidinium tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro mouse lymphoma assay, and in vivo rat bone marrow micronucleus assay.
No evidence of impairment of fertility was observed in male and female rats at subcutaneous doses up to 180 mcg/kg/day and at inhaled doses up to 294 mcg/kg/day, respectively (approximately 100 and 50 times, respectively, the MRHDID for adults on an AUC basis).
Vilanterol
In a 2-year carcinogenicity study in mice, vilanterol caused a statistically significant increase in ovarian tubulostromal adenomas in females at an inhaled dose of 29,500 mcg/kg/day (approximately 7,800 times the MRHDID for adults on an AUC basis). No increase in tumors was seen at an inhaled dose of 615 mcg/kg/day (approximately 210 times the MRHDID for adults on an AUC basis).
In a 2-year carcinogenicity study in rats, vilanterol caused statistically significant increases in mesovarian leiomyomas in females and shortening of the latency of pituitary tumors at inhaled doses greater than or equal to 84.4 mcg/kg/day (greater than or equal to approximately 20 times the MRHDID for adults on an AUC basis). No tumors were seen at an inhaled dose of 10.5 mcg/kg/day (approximately equal to the MRHDID for adults on an AUC basis).
These tumor findings in rodents are similar to those reported previously for other beta-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Vilanterol tested negative in the following genotoxicity assays: the in vitro Ames assay, in vivo rat bone marrow micronucleus assay, in vivo rat unscheduled DNA synthesis (UDS) assay, and in vitro Syrian hamster embryo (SHE) cell assay. Vilanterol tested equivocal in the in vitro mouse lymphoma assay.
No evidence of impairment of fertility was observed in male and female rats at inhaled vilanterol doses up to 31,500 and 37,100 mcg/kg/day, respectively (both approximately 5,490 times the MRHDID based on AUC).
-
14 CLINICAL STUDIESThe safety and effectiveness of ANORO ELLIPTA were evaluated in a clinical development program that included 6 dose-ranging trials, 4 lung function trials of 6 months’ duration (2 placebo ...
The safety and effectiveness of ANORO ELLIPTA were evaluated in a clinical development program that included 6 dose-ranging trials, 4 lung function trials of 6 months’ duration (2 placebo controlled and 2 active controlled), two 12-week crossover trials, and a 12-month long‑term safety trial. The efficacy of ANORO ELLIPTA is based primarily on the dose-ranging trials in 1,908 subjects with COPD or asthma [see Clinical Studies (14.1)] and the 2 placebo-controlled confirmatory trials, with additional support from the 2 active-controlled and 2 crossover trials in 5,388 subjects with COPD, including chronic bronchitis and/or emphysema [see Clinical Studies (14.2)]. Evidence of efficacy for ANORO ELLIPTA on COPD exacerbations was established by the efficacy of the umeclidinium component as part of a fixed-dose combination with an ICS/LABA, as assessed in a 12-month trial in 10,355 subjects [see Clinical Studies (14.2)].
14.1 Dose-Ranging Trials
Dose selection for ANORO ELLIPTA in COPD was based on dose-ranging trials for the individual components, vilanterol and umeclidinium. Based on the findings from these studies, once-daily doses of umeclidinium/vilanterol 62.5/25 mcg and umeclidinium/vilanterol 125/25 mcg were evaluated in the confirmatory COPD trials. ANORO ELLIPTA is not indicated for asthma.
Umeclidinium
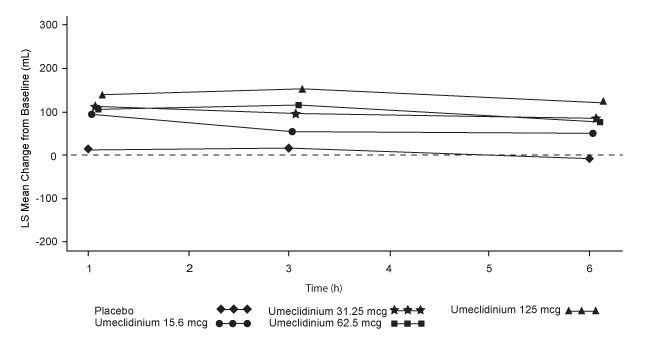
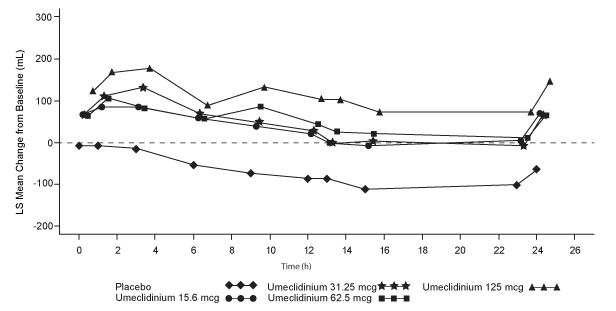
Dose selection for umeclidinium in COPD was supported by a 7-day, randomized, double-blind, placebo-controlled, crossover trial evaluating 4 doses of umeclidinium (15.6 to 125 mcg) or placebo dosed once daily in the morning in 163 subjects with COPD. A dose ordering was observed, with the 62.5- and 125-mcg doses demonstrating larger improvements in FEV1 over 24 hours compared with the lower doses of 15.6 and 31.25 mcg (Figure 3).
The differences in trough FEV1 from baseline after 7 days for placebo and the 15.6-, 31.25-, 62.5-, and 125-mcg doses were -74 mL (95% CI: -118, -31), 38 mL (95% CI: -6, 83), 27 mL (95% CI: -18, 72), 49 mL (95% CI: 6, 93), and 109 mL (95% CI: 65, 152), respectively. Two additional dose-ranging trials in subjects with COPD demonstrated minimal additional benefit at doses above 125 mcg. The dose-ranging results supported the evaluation of 2 doses of umeclidinium, 62.5 and 125 mcg, in the confirmatory COPD trials to further assess dose response.
Evaluations of dosing interval by comparing once- and twice-daily dosing supported selection of a once-daily dosing interval for further evaluation in the confirmatory COPD trials.
Figure 3. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (mL) on Days 1 and 7
Day 1
Day 7
Vilanterol
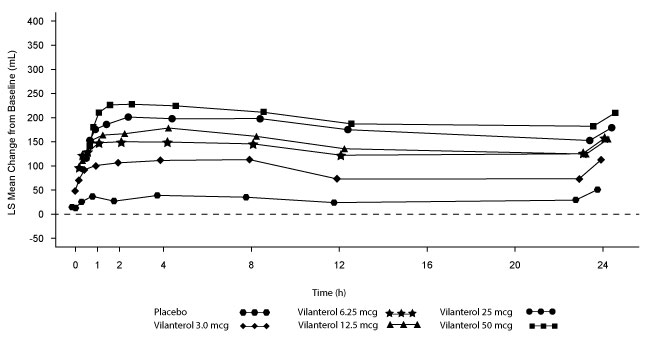
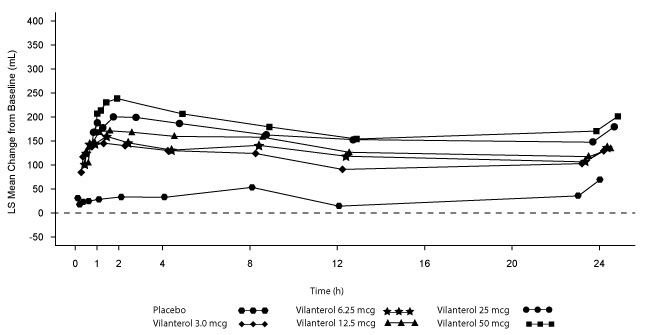
Dose selection for vilanterol in COPD was supported by a 28-day, randomized, double-blind, placebo-controlled, parallel-group trial evaluating 5 doses of vilanterol (3 to 50 mcg) or placebo dosed in the morning in 602 subjects with COPD. Results demonstrated dose-related increases from baseline in FEV1 at Day 1 and Day 28 (Figure 4).
Figure 4. Least Squares (LS) Mean Change from Baseline in Postdose Serial FEV1 (0-24 h) (mL) on Days 1 and 28
Day 1
Day 28
The differences in trough FEV1 after Day 28 from baseline for placebo and the 3-, 6.25-, 12.5-, 25-, and 50-mcg doses were 29 mL (95% CI: -8, 66), 120 mL (95% CI: 83, 158), 127 mL (95% CI: 90, 164), 138 mL (95% CI: 101, 176), 166 mL (95% CI: 129, 203), and 194 mL (95% CI: 156, 231), respectively. These results supported the evaluation of vilanterol 25 mcg in the confirmatory trials for COPD.
Dose-ranging trials in subjects with asthma evaluated doses from 3 to 50 mcg and 12.5 mcg once-daily versus 6.25 mcg twice-daily dosing frequency. The results supported the selection of the vilanterol 25 mcg once-daily dose for further evaluation in the confirmatory trials for COPD.
Close14.2 Confirmatory Trials
Lung Function
The clinical development program for ANORO ELLIPTA included two 6-month, randomized, double-blind, placebo-controlled, parallel-group trials; two 6-month active-controlled trials; and two 12-week crossover trials in subjects with COPD designed to evaluate the efficacy of ANORO ELLIPTA on lung function. The 6-month trials treated 4,733 subjects that had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking ≥10 pack-years, had a post-albuterol FEV1 ≤70% of predicted normal values, had a ratio of FEV1/FVC of <0.7, and had a Modified Medical Research Council (mMRC) score ≥2. Of the 4,713 subjects included in the efficacy analysis, 68% were male and 84% were white. They had a mean age of 63 years and an average smoking history of 45 pack-years, with 50% identified as current smokers. At screening, the mean postbronchodilator percent predicted FEV1 was 48% (range: 13% to 76%), the mean postbronchodilator FEV1/FVC ratio was 0.47 (range: 0.13 to 0.78), and the mean percent reversibility was 14% (range: -36% to 109%).
Trial 1 (NCT01313650) evaluated ANORO ELLIPTA (umeclidinium/vilanterol 62.5/25 mcg), umeclidinium 62.5 mcg, vilanterol 25 mcg, and placebo. The primary endpoint was change from baseline in trough (predose) FEV1 at Day 169 (defined as the mean of the FEV1 values obtained at 23 and 24 hours after the previous dose on Day 168) compared with placebo, umeclidinium 62.5 mcg, and vilanterol 25 mcg. The comparison of ANORO ELLIPTA with umeclidinium 62.5 mcg and vilanterol 25 mcg was assessed to evaluate the contribution of the individual comparators to ANORO ELLIPTA. ANORO ELLIPTA demonstrated a larger increase in mean change from baseline in trough (predose) FEV1 relative to placebo, umeclidinium 62.5 mcg, and vilanterol 25 mcg (Table 2).
Table 2. Least Squares Mean Change from Baseline in Trough FEV1 (mL) at Day 169 in the Intent-to-Treat Population (Trial 1) n = Number in intent-to-treat population.
a The umeclidinium and vilanterol comparators used the same inhaler and excipients as ANORO ELLIPTA.Treatment
n
Trough FEV1 (mL) at Day 169
Difference from
Placebo
(95% CI)
n = 280
Umeclidinium
62.5 mcga
(95% CI)
n = 418
Vilanterol
25 mcga
(95% CI)
n = 421
ANORO ELLIPTA
413
167
(128, 207)
52
(17, 87)
95
(60, 130)
Trial 2 (NCT01313637) had a similar study design as Trial 1 but evaluated umeclidinium/vilanterol 125/25 mcg, umeclidinium 125 mcg, vilanterol 25 mcg, and placebo. Results for umeclidinium/vilanterol 125/25 mcg in Trial 2 were similar to those observed for ANORO ELLIPTA in Trial 1.
Results from the 2 active-controlled trials and the two 12-week trials provided additional support for the efficacy of ANORO ELLIPTA in terms of change from baseline in trough FEV1 compared with the single-ingredient comparators and placebo.
Serial spirometric evaluations throughout the 24-hour dosing interval were performed in a subset of subjects (n = 197) at Days 1, 84, and 168 in Trial 1. Results from Trial 1 at Day 1 and Day 168 are shown in Figure 5.
Figure 5. Least Squares (LS) Mean Change from Baseline in FEV1 (mL) over Time (0-24 h) on Days 1 and 168 (Trial 1 Subset Population)
Day 1
Day 168
The peak FEV1 was defined as the maximum FEV1 recorded within 6 hours after the dose of trial medicine on Days 1, 28, 84, and 168 (measurements recorded at 15 and 30 minutes and 1, 3, and 6 hours). The mean peak FEV1 improvement from baseline for ANORO ELLIPTA compared with placebo at Day 1 and at Day 168 was 167 and 224 mL, respectively. The median time to onset on Day 1, defined as a 100-mL increase from baseline in FEV1, was 27 minutes in subjects receiving ANORO ELLIPTA.
Exacerbations
In Trial 6 (NCT02164513), a total of 10,355 subjects with COPD with a history of 1 or more moderate or severe exacerbations in the prior 12 months were randomized (1:2:2) to receive ANORO ELLIPTA (n = 2,070), fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 mcg (n = 4,151), or fluticasone furoate/vilanterol 100/25 mcg (n = 4,134) administered once daily in a 12-month trial. The population demographics across all treatments were: mean age of 65 years, 77% white, 66% male, and an average smoking history of 46.6 pack-years, with 35% identified as current smokers. At trial entry, the most common COPD medications were ICS + anticholinergic + LABA (34%), ICS + LABA (26%), anticholinergic + LABA (8%), and anticholinergic (7%). The mean postbronchodilator percent predicted FEV1 was 46% (standard deviation: 15%), the mean postbronchodilator FEV1/FVC ratio was 0.47 (standard deviation: 0.12), and the mean percent reversibility was 10% (range: -59% to 125%).
The primary endpoint was annual rate of on-treatment moderate and severe exacerbations in subjects treated with fluticasone furoate/umeclidinium/vilanterol compared with the fixed-dose combinations of fluticasone furoate/vilanterol and ANORO ELLIPTA. Exacerbations were defined as worsening of 2 or more major symptoms (dyspnea, sputum volume, and sputum purulence) or worsening of any 1 major symptom together with any 1 of the following minor symptoms: sore throat, colds (nasal discharge and/or nasal congestion), fever without other cause, and increased cough or wheeze for at least 2 consecutive days. Exacerbations were considered to be of moderate severity if treatment with systemic corticosteroids and/or antibiotics was required and were considered to be severe if resulted in hospitalization or death.
Contribution of Umeclidinium on COPD Exacerbations: Evidence of efficacy for ANORO ELLIPTA on COPD exacerbations was established by the efficacy of the umeclidinium component of fluticasone furoate/umeclidinium/vilanterol in Trial 6. Treatment with fluticasone furoate/umeclidinium/vilanterol statistically significantly reduced the on-treatment annual rate of moderate/severe exacerbations by 15% compared with fluticasone furoate/vilanterol (Table 3). A reduction in risk of on-treatment moderate/severe exacerbation (as measured by time to first) was also observed for the same comparison. The benefit of umeclidinium on exacerbations is not expected to diminish when combined with vilanterol in ANORO ELLIPTA.
ANORO ELLIPTA and COPD Exacerbations: In Trial 6, the primary efficacy analysis of the rate of moderate/severe exacerbations, treatment with fluticasone furoate/umeclidinium/vilanterol statistically significantly reduced the on-treatment annual rate of moderate/severe exacerbations by 25% compared with ANORO ELLIPTA (Table 3).
Table 3. Moderate and Severe Chronic Obstructive Pulmonary Disease Exacerbations (Trial 6)a FF/UMEC/VI = Fluticasone furoate/umeclidinium/vilanterol 100/62.5/25 mcg, FF/VI = Fluticasone furoate/vilanterol 100/25 mcg, ANORO ELLIPTA = Umeclidinium/vilanterol 62.5/25 mcg.
a On-treatment analyses excluded exacerbation data collected after discontinuation of study treatment.Treatment
n
Mean Annual Rate
(exacerbations/year)
FF/UMEC/VI Rate Ratio vs. Comparator
(95% CI)
% Reduction in Exacerbation Rate
(95% CI)
P Value
FF/UMEC/VI
4,145
0.91
FF/VI
4,133
1.07
0.85
(0.80, 0.90)
15
(10, 20)
P<0.001
ANORO ELLIPTA
2,069
1.21
0.75
(0.70, 0.81)
25
(19, 30)
P<0.001
-
16 HOW SUPPLIED/STORAGE AND HANDLINGANORO ELLIPTA is supplied as a disposable light grey and red plastic inhaler containing 2 foil strips, each with 30 blisters (or 7 blisters for the institutional pack). One strip contains ...
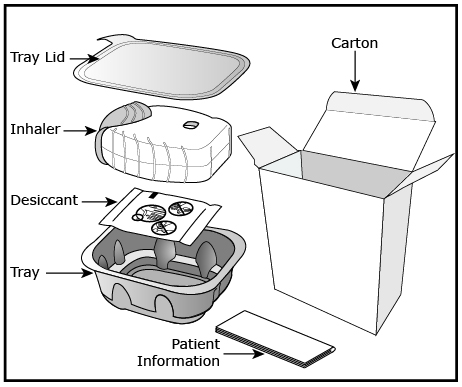
ANORO ELLIPTA is supplied as a disposable light grey and red plastic inhaler containing 2 foil strips, each with 30 blisters (or 7 blisters for the institutional pack). One strip contains umeclidinium (62.5 mcg per blister), and the other strip contains vilanterol (25 mcg per blister). A blister from each strip is used to create 1 dose. The inhaler is packaged in a moisture-protective foil tray with a desiccant and a peelable lid in the following packs:
NDC 0173-0869-10 30 inhalations (60 blisters)
NDC 0173-0869-06 7 inhalations (14 blisters), institutional pack
Store at room temperature between 68°F and 77°F (20°C and 25°C); excursions permitted from 59°F to 86°F (15°C to 30°C) [See USP Controlled Room Temperature]. Store in a dry place away from direct heat or sunlight. Keep out of reach of children.
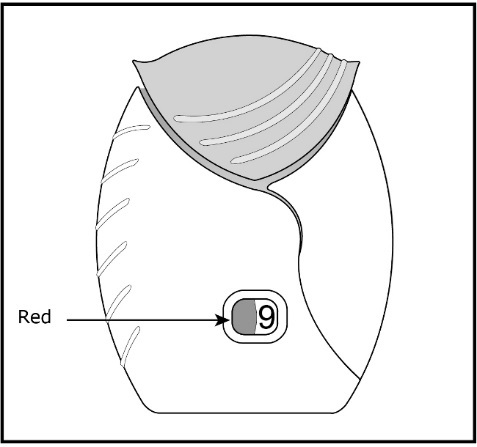
ANORO ELLIPTA should be stored inside the unopened moisture-protective foil tray and only removed from the tray immediately before initial use. Discard ANORO ELLIPTA 6 weeks after opening the foil tray or when the counter reads “0” (after all blisters have been used), whichever comes first. The inhaler is not reusable. Do not attempt to take the inhaler apart.
Close -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Serious Asthma-Related Events - ANORO ELLIPTA is not indicated for the treatment of ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Asthma-Related Events
ANORO ELLIPTA is not indicated for the treatment of asthma. Inform patients that LABAs, such as vilanterol (one of the active ingredients in ANORO ELLIPTA), when used alone (without ICS) for asthma increase the risk of asthma-related hospitalization or asthma-related death. [See Warnings and Precautions (5.1).]
Not for Acute Symptoms
Inform patients that ANORO ELLIPTA is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise patients to treat acute symptoms with an inhaled, short-acting beta2-agonist such as albuterol. Provide patients with such medication and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
- •
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- •
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- •
- Significant decrease in lung function as outlined by the physician
Tell patients they should not stop therapy with ANORO ELLIPTA without physician/provider guidance since symptoms may recur after discontinuation. [See Warnings and Precautions (5.2).]
Do Not Use Additional Long-acting Beta2-agonists
Instruct patients not to use other LABAs for COPD. [See Warnings and Precautions (5.3).]
Paradoxical Bronchospasm
As with other inhaled medicines, ANORO ELLIPTA can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue ANORO ELLIPTA and contact their healthcare provider right away. [See Warnings and Precautions (5.5).]
Risks Associated with Beta-agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. Instruct patients to consult a healthcare practitioner immediately should any of these signs and symptoms develop. [See Warnings and Precautions (5.7).]
Worsening of Narrow-Angle Glaucoma
Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develop. [See Warnings and Precautions (5.9).]
Worsening of Urinary Retention
Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately if any of these signs or symptoms develop. [See Warnings and Precautions (5.10).]
Trademarks are owned by or licensed to the GSK group of companies.
ANORO ELLIPTA was developed in collaboration with Innoviva.GlaxoSmithKline
Durham, NC 27701©2023 GSK group of companies or its licensor.
ANR:12PI
Close -
PATIENT PACKAGE INSERTPATIENT INFORMATION - ANORO ELLIPTA (a-NOR-oh e-LIP-ta) (umeclidinium and vilanterol inhalation powder) for oral inhalation use - What is ANORO ELLIPTA? • ANORO ELLIPTA combines ...
PATIENT INFORMATION
ANORO ELLIPTA (a-NOR-oh e-LIP-ta)
(umeclidinium and vilanterol inhalation powder)
for oral inhalation use
What is ANORO ELLIPTA?
- •
- ANORO ELLIPTA combines 2 medicines in 1 inhaler, an anticholinergic medicine (umeclidinium) and a long‑acting beta2-adrenergic agonist (LABA) medicine (vilanterol).
- o
- Anticholinergic medicines such as umeclidinium and LABA medicines such as vilanterol help the muscles around the airways in your lungs stay relaxed to prevent symptoms such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- •
- ANORO ELLIPTA is not used to relieve sudden breathing problems and will not replace a rescue inhaler.
- •
- ANORO ELLIPTA is a prescription medicine used long term (chronic) to treat people with chronic obstructive pulmonary disease (COPD). COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- •
- ANORO ELLIPTA is used as 1 inhalation 1 time each day to improve symptoms of COPD for better breathing and to reduce the number of flare-ups (the worsening of your COPD symptoms for several days).
- •
- ANORO ELLIPTA is not for the treatment of asthma. It is not known if ANORO ELLIPTA is safe and effective in people with asthma.
ANORO ELLIPTA should not be used in children.
It is not known if ANORO ELLIPTA is safe and effective in children.
Do not use ANORO ELLIPTA if you:
- •
- have a severe allergy to milk proteins. Ask your healthcare provider if you are not sure.
- •
- are allergic to umeclidinium, vilanterol, or any of the ingredients in ANORO ELLIPTA. See the end of this Patient Information for a complete list of ingredients in ANORO ELLIPTA.
- •
- have asthma.
Before using ANORO ELLIPTA, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have heart problems.
- •
- have high blood pressure.
- •
- have seizures.
- •
- have thyroid problems.
- •
- have diabetes.
- •
- have liver problems.
- •
- have eye problems such as glaucoma. ANORO ELLIPTA may make your glaucoma worse.
- •
- are allergic to milk proteins.
- •
- have prostate or bladder problems, or problems passing urine. ANORO ELLIPTA may make these problems worse.
- •
- are pregnant or plan to become pregnant. It is not known if ANORO ELLIPTA may harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if the medicines in ANORO ELLIPTA pass into your breast milk and if they can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. ANORO ELLIPTA and certain other medicines may interact with each other. This may cause serious side effects.
Especially tell your healthcare provider if you take:
- •
- anticholinergics (including tiotropium, ipratropium, aclidinium)
- •
- atropine
- •
- other LABAs (including salmeterol, formoterol, arformoterol, olodaterol, and indacaterol)
- •
- antifungal or anti-HIV medicines
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use ANORO ELLIPTA?
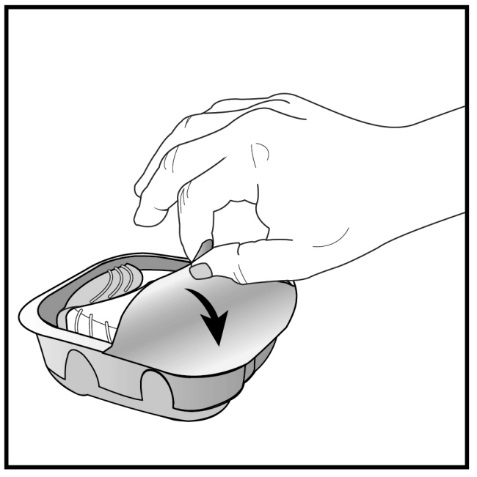
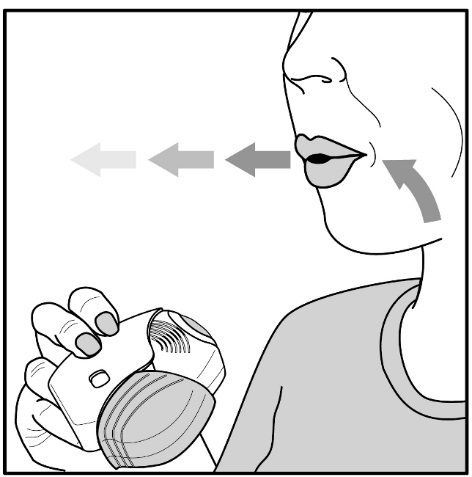
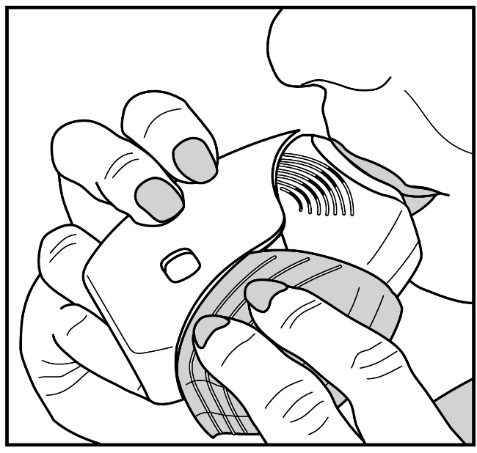
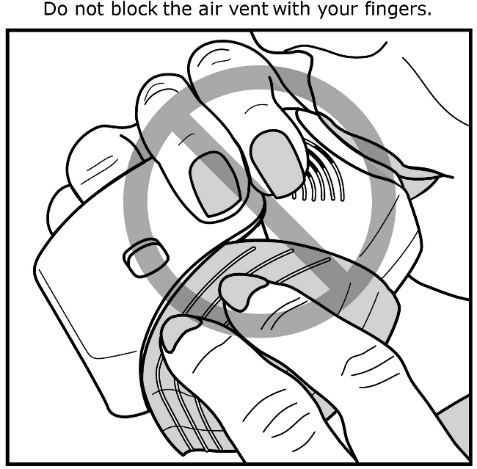
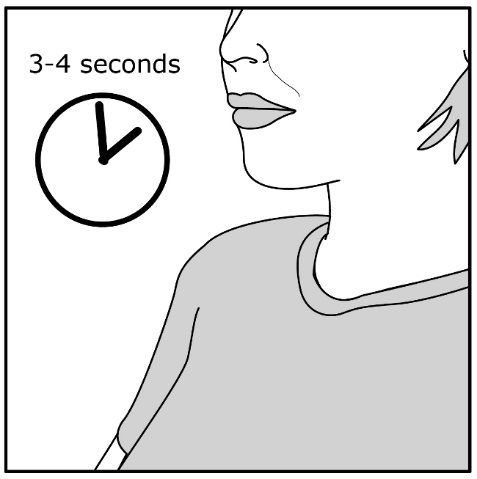
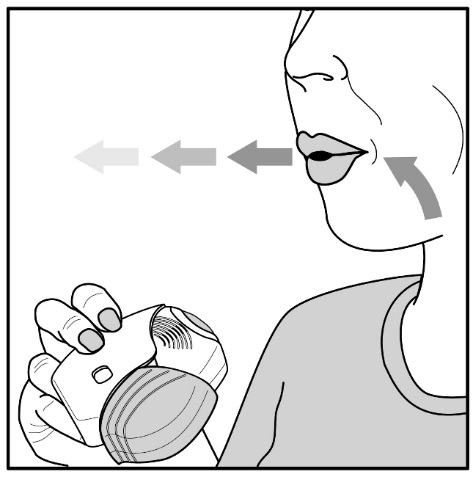
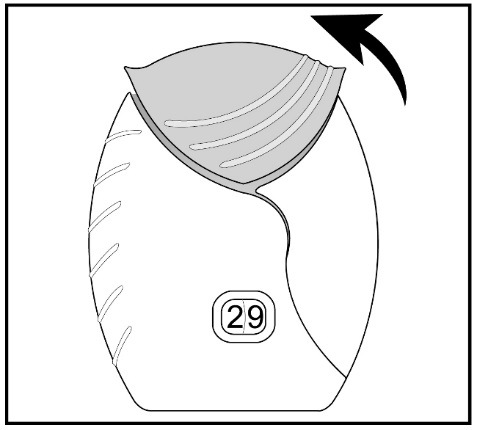
Read the step-by-step instructions for using ANORO ELLIPTA at the end of this Patient Information.
- •
- Do not use ANORO ELLIPTA unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- •
- Use ANORO ELLIPTA exactly as your healthcare provider tells you to use it. Do not use ANORO ELLIPTA more often than prescribed.
- •
- Use 1 inhalation of ANORO ELLIPTA 1 time each day. Use ANORO ELLIPTA at the same time each day.
- •
- If you miss a dose of ANORO ELLIPTA, take it as soon as you remember. Do not take more than 1 inhalation per day. Take your next dose at your usual time. Do not take 2 doses at 1 time.
- •
- If you take too much ANORO ELLIPTA, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- •
- Do not use other medicines that contain a LABA or an anticholinergic for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA or anticholinergic medicines.
- •
- Do not stop using ANORO ELLIPTA unless told to do so by your healthcare provider because your symptoms might get worse. Your healthcare provider will change your medicines as needed.
- •
- ANORO ELLIPTA does not relieve sudden symptoms of COPD and you should not take extra doses of ANORO ELLIPTA to relieve these sudden symptoms. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- •
- Call your healthcare provider or get medical care right away if:
- o
- your breathing problems get worse.
- o
- you need to use your rescue inhaler more often than usual.
- o
- your rescue inhaler does not work as well to relieve your symptoms.
What are the possible side effects of ANORO ELLIPTA?
ANORO ELLIPTA can cause serious side effects, including:
- •
-
serious problems in people with asthma. People with asthma who take LABA medicines, such as vilanterol (one of the medicines in ANORO ELLIPTA), without also using a medicine called an inhaled corticosteroid, have an increased risk of serious problems from asthma, including death.
- o
- Call your healthcare provider if breathing problems worsen over time while using ANORO ELLIPTA. You may need a different treatment.
- o
- Get emergency medical care if:
- •
- your breathing problems worsen quickly.
- •
- you use your rescue inhaler medicine, but it does not relieve your breathing problems.
- •
- COPD symptoms that get worse over time. If your COPD symptoms worsen over time, do not increase your dose of ANORO ELLIPTA; instead call your healthcare provider.
- •
- symptoms of using too much of a LABA medicine, including:
- o
- chest pain
- o
- fast or irregular heartbeat
- o
- tremor
- o
- increased blood pressure
- o
- headache
- o
- nervousness
- •
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop using ANORO ELLIPTA and call your healthcare provider right away.
- •
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- o
- rash
- o
- hives
- o
- swelling of your face, mouth, and tongue
- o
- breathing problems
- •
- effects on heart.
- o
- increased blood pressure
- o
- a fast or irregular heartbeat, awareness of heartbeat
- o
- chest pain
- •
- effects on nervous system.
- o
- tremor
- o
- nervousness
- •
- new or worsening eye problems including acute narrow-angle glaucoma. You should have regular eye exams while using ANORO ELLIPTA. Acute narrow-angle glaucoma can cause permanent loss of vision if not treated. Symptoms of acute narrow-angle glaucoma may include:
- o
- eye pain or discomfort
- o
- blurred vision
- o
- red eyes
- o
- nausea or vomiting
- o
- seeing halos or bright colors around lights
- If you have these symptoms, call your healthcare provider right away before taking another dose.
- •
- urinary retention. People who take ANORO ELLIPTA may develop new or worse urinary retention. Symptoms of urinary retention may include:
- o
- difficulty urinating
- o
- urinating frequently
- o
- painful urination
- o
- urination in a weak stream or drips
- If you have these symptoms of urinary retention, stop taking ANORO ELLIPTA and call your healthcare provider right away before taking another dose.
- •
- changes in laboratory blood values, including high levels of blood sugar (hyperglycemia) and low levels of potassium (hypokalemia).
Common side effects of ANORO ELLIPTA include:
- o
- sore throat
- o
- common cold symptoms
- o
- pain in your arms or legs
- o
- chest pain
- o
- sinus infection
- o
- constipation
- o
- muscle spasms
- o
- lower respiratory infection
- o
- diarrhea
- o
- neck pain
These are not all the possible side effects of ANORO ELLIPTA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ANORO ELLIPTA?
- •
- Store ANORO ELLIPTA at room temperature between 68°F and 77°F (20°C and 25°C). Keep in a dry place away from heat and sunlight.
- •
- Store ANORO ELLIPTA in the unopened tray and only open when ready for use.
- •
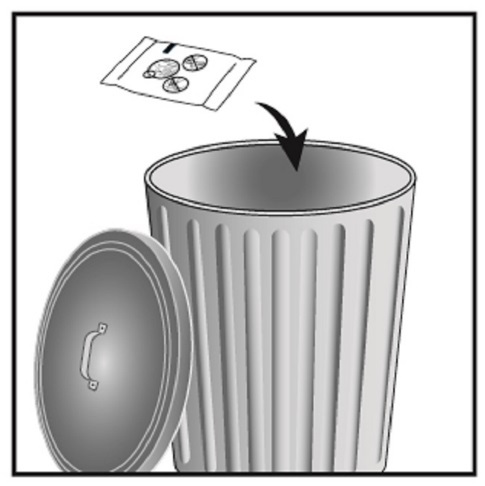
- Safely throw away ANORO ELLIPTA in the trash 6 weeks after you open the tray or when the counter reads “0”, whichever comes first. Write the date you open the tray on the label on the inhaler.
- •
- Keep ANORO ELLIPTA and all medicines out of the reach of children.
General information about the safe and effective use of ANORO ELLIPTA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ANORO ELLIPTA for a condition for which it was not prescribed. Do not give ANORO ELLIPTA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about ANORO ELLIPTA that is written for health professionals.
What are the ingredients in ANORO ELLIPTA?
Active ingredients: umeclidinium, vilanterol
Inactive ingredients: lactose monohydrate (contains milk proteins), magnesium stearate
For more information about ANORO ELLIPTA, call 1-888-825-5249.
Trademarks are owned by or licensed to the GSK group of companies.
ANORO ELLIPTA was developed in collaboration with Innoviva.
GlaxoSmithKline, Durham, NC 27701
©2022 GSK group of companies or its licensor.
ANR:10PIL
- This Patient Information has been approved by the U.S. Food and Drug Administration Revised: October 2022
Close- This Instructions for Use has been approved by the U.S. Food and Drug Administration Revised: October 2022
-
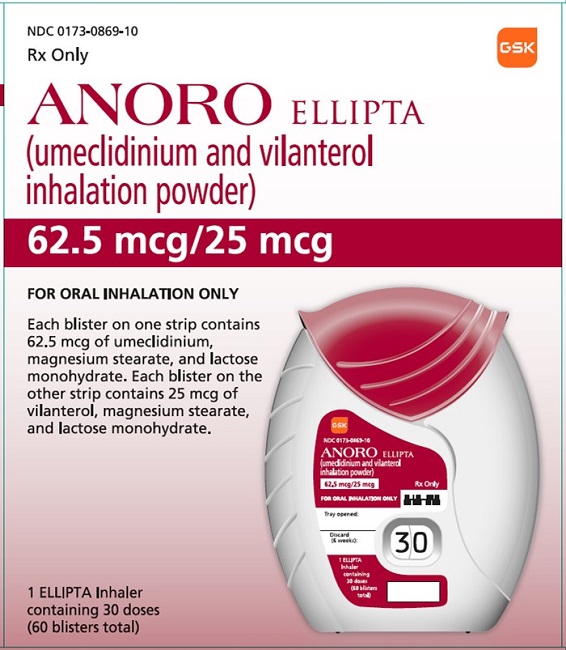
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 0173-0869-10 - ANORO ELLIPTA - (umeclidinium and vilanterol inhalation powder) 62.5 mcg/25 mcg - Rx Only - FOR ORAL INHALATION ONLY - Each blister on one strip ...
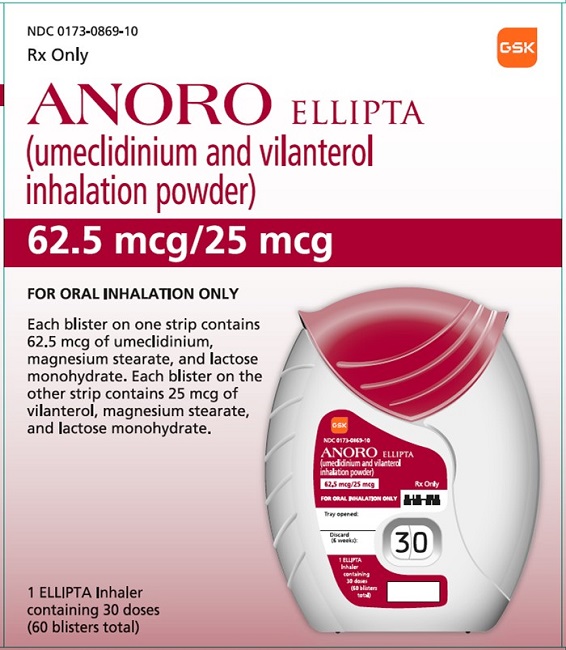
PRINCIPAL DISPLAY PANEL
NDC 0173-0869-10
ANORO ELLIPTA
(umeclidinium and vilanterol inhalation powder)
62.5 mcg/25 mcg
Rx Only
FOR ORAL INHALATION ONLY
Each blister on one strip contains 62.5 mcg of umeclidinium, magnesium stearate, and lactose monohydrate. Each blister on the other strip contains 25 mcg of vilanterol, magnesium stearate, and lactose monohydrate.
1 ELLIPTA Inhaler containing 30 doses (60 blisters total)
GSK
©2023 GSK group of companies or its licensor.
- 62000000084535 Rev.1/23
-
INGREDIENTS AND APPEARANCEProduct Information
ANORO ELLIPTA umeclidinium bromide and vilanterol trifenatate powder Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0173-0869 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UMECLIDINIUM BROMIDE (UNII: 7AN603V4JV) (UMECLIDINIUM - UNII:GE2T1418SV) UMECLIDINIUM 62.5 ug VILANTEROL TRIFENATATE (UNII: 40AHO2C6DG) (VILANTEROL - UNII:028LZY775B) VILANTEROL 25 ug Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0173-0869-10 1 in 1 CARTON 01/31/2014 1 1 in 1 TRAY 1 30 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:0173-0869-06 1 in 1 CARTON 01/31/2014 2 1 in 1 TRAY 2 7 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:0173-0869-61 1 in 1 CARTON 01/31/2014 10/31/2024 3 1 in 1 TRAY 3 7 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203975 01/31/2014
CloseLabeler - GlaxoSmithKline LLC (167380711)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ANORO ELLIPTA- umeclidinium bromide and vilanterol trifenatate powder
Number of versions: 27
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jan 1, 2025 | 28 (current) | download |
| Mar 8, 2024 | 27 | download |
| Jun 23, 2023 | 26 | download |
| Nov 9, 2022 | 25 | download |
| Nov 24, 2021 | 24 | download |
| Dec 30, 2020 | 23 | download |
| Aug 27, 2020 | 22 | download |
| Jan 8, 2020 | 21 | download |
| Aug 5, 2019 | 20 | download |
| Jul 2, 2019 | 19 | download |
| Jun 24, 2019 | 18 | download |
| Jun 6, 2019 | 17 | download |
| Jan 16, 2019 | 16 | download |
| Nov 6, 2017 | 15 | download |
| Jul 19, 2017 | 14 | download |
| Jun 22, 2017 | 13 | download |
| Mar 28, 2017 | 12 | download |
| Jan 17, 2017 | 11 | download |
| Mar 9, 2016 | 10 | download |
| Jan 19, 2016 | 9 | download |
| Dec 16, 2015 | 8 | download |
| Jan 22, 2015 | 7 | download |
| Jan 16, 2015 | 6 | download |
| Sep 3, 2014 | 5 | download |
| Jun 25, 2014 | 4 | download |
| May 13, 2014 | 3 | download |
| Jan 31, 2014 | 2 | download |
RxNorm
ANORO ELLIPTA- umeclidinium bromide and vilanterol trifenatate powder
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1487519 | umeclidinium/vilanterol 62.5/25 MCG/INHAL Dry Powder Inhaler, 7 Blisters | PSN |
| 2 | 1487519 | 7 ACTUAT umeclidinium 0.0625 MG/ACTUAT / vilanterol 0.025 MG/ACTUAT Dry Powder Inhaler | SCD |
| 3 | 1487519 | umeclidinium 0.0625 MG/ACTUAT / vilanterol 0.025 MG/ACTUAT 7 ACTUAT Dry Powder Inhaler | SY |
| 4 | 1487524 | ANORO Ellipta 62.5/25 MCG/INHAL Dry Powder Inhaler, 7 Blisters | PSN |
| 5 | 1487524 | 7 ACTUAT umeclidinium 0.0625 MG/ACTUAT / vilanterol 0.025 MG/ACTUAT Dry Powder Inhaler [Anoro] | SBD |
| 6 | 1487524 | Anoro Ellipta (umeclidinium 62.5 MCG / vilanterol 25 MCG) per ACTUAT Dry Powder Inhaler, 7 ACTUAT | SY |
| 7 | 1487527 | umeclidinium/vilanterol 62.5/25 MCG/INHAL Dry Powder Inhaler, 30 Blisters | PSN |
| 8 | 1487527 | 30 ACTUAT umeclidinium 0.0625 MG/ACTUAT / vilanterol 0.025 MG/ACTUAT Dry Powder Inhaler | SCD |
| 9 | 1487527 | umeclidinium 0.0625 MG/ACTUAT / vilanterol 0.025 MG/ACTUAT 30 ACTUAT Dry Powder Inhaler | SY |
| 10 | 1487528 | ANORO Ellipta 62.5/25 MCG/INHAL Dry Powder Inhaler, 30 Blisters | PSN |
| 11 | 1487528 | 30 ACTUAT umeclidinium 0.0625 MG/ACTUAT / vilanterol 0.025 MG/ACTUAT Dry Powder Inhaler [Anoro] | SBD |
| 12 | 1487528 | Anoro Ellipta (umeclidinium 62.5 MCG / vilanterol 25 MCG) per ACTUAT Dry Powder Inhaler, 30 ACTUAT | SY |
Get Label RSS Feed for this Drug
ANORO ELLIPTA- umeclidinium bromide and vilanterol trifenatate powder
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ANORO ELLIPTA- umeclidinium bromide and vilanterol trifenatate powder
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0173-0869-06 |
| 2 | 0173-0869-10 |
| 3 | 0173-0869-61 |