Label: ANDEXXA- andexanet alfa injection, powder, lyophilized, for solution
- NDC Code(s): 0310-3200-01, 0310-3200-04, 0310-3200-05
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ANDEXXA safely and effectively. See Full Prescribing Information for ANDEXXA. ANDEXXA® (coagulation factor Xa (recombinant) ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: THROMBOEMBOLIC RISKS, ISCHEMIC RISKS, CARDIAC ARREST, AND SUDDEN DEATHS
Treatment with ANDEXXA has been associated with serious and life-threatening adverse events, including: (5.1)
- •
- Arterial and venous thromboembolic events
- •
- Ischemic events, including myocardial infarction and ischemic stroke
- •
- Cardiac arrest
- •
- Sudden deaths
Monitor for thromboembolic events and initiate anticoagulation when medically appropriate. Monitor for symptoms and signs that precede cardiac arrest and provide treatment as needed.
Close -
1 INDICATIONS AND USAGEANDEXXA is indicated for patients treated with rivaroxaban or apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. This indication is approved ...
-
2 DOSAGE AND ADMINISTRATIONFor intravenous (IV) use only. 2.1 Dose - There are two dosing regimens (see Table 1). The safety and efficacy of an additional dose have not been established. Table 1: ANDEXXA Dosing ...
-
3 DOSAGE FORMS AND STRENGTHSANDEXXA is available as a white to off-white lyophilized powder in single-use vials of 200 mg of coagulation factor Xa (recombinant), inactivated-zhzo.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Thromboembolic and Ischemic Risks - The thromboembolic and ischemic risks were assessed in 419 bleeding subjects in the ANNEXA-4 study who received ANDEXXAand were treated with apixaban or ...
-
6 ADVERSE REACTIONSThe most common adverse reactions (≥ 5%) in bleeding subjects receiving ANDEXXA were urinary tract infections and pneumonia. 6.1 Clinical Trials Experience - Because clinical trials are conducted ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of ANDEXXA in pregnant women to inform patients of associated risks. Animal reproductive and developmental ...
-
11 DESCRIPTIONANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a sterile, white to off-white lyophilized powder available in single-use vials. Each 200 mg vial delivers 200 mg of coagulation ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Coagulation factor Xa (recombinant), inactivated-zhzo exerts its procoagulant effect by binding and sequestering the FXa inhibitors, rivaroxaban and apixaban. Another ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No animal studies were performed to evaluate the effects of ANDEXXA on carcinogenesis, mutagenesis, or impairment of fertility.
-
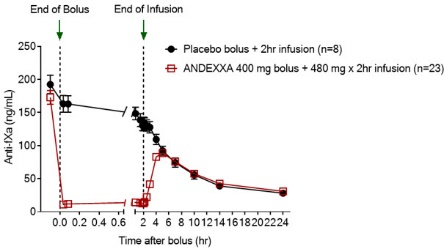
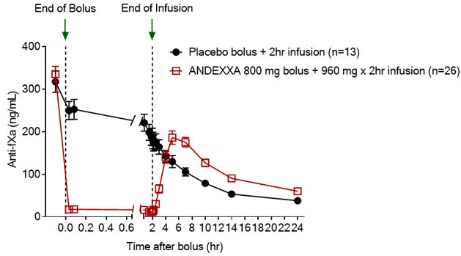
14 CLINICAL STUDIESThe safety and efficacy of ANDEXXA were evaluated in two prospective, randomized, placebo-controlled - studies, conducted in healthy volunteers (Study 1 ANNEXA-A; Study 2 ANNEXA-R). Both studies ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ANDEXXA (coagulation factor Xa (recombinant), inactivated-zhzo) is a white to off-white lyophilized cake or powder supplied as single-use vials in a carton. ANDEXXA is not made ...
-
17 PATIENT COUNSELING INFORMATIONInform patients that reversing FXa inhibitor therapy increases the risk of thromboembolic events. Arterial and venous thromboembolic events, ischemic events, cardiac events, and sudden death were ...
-
SPL UNCLASSIFIED SECTIONManufactured by: AstraZeneca AB - Södertälje, Sweden SE-15185 - Distributed by: AstraZeneca Pharmaceuticals LP - Wilmington, DE 19850 - U.S. License No. 2059 - Product of Spain - ANDEXXA is a registered ...
-
PRINCIPAL DISPLAY PANEL - 200 mg Vial CartonNDC 0310-3200-04 Rx Only - ANDEXXA® 200mg/vial - coagulation factor Xa - (recombinant), inactivated-zhzo - Lyophilized powder for solution. For intravenous use only. Four 200 mg single-dose vials ...
-
INGREDIENTS AND APPEARANCEProduct Information