Label: AMZEEQ- minocycline aerosol, foam
- NDC Code(s): 69489-201-03, 69489-201-30
- Packager: Journey Medical Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMZEEQ safely and effectively. See full prescribing information for AMZEEQ. AMZEEQ - ®(minocycline) topical foam - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAMZEEQ is indicated for the topical treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in adults and pediatric patients 9 years of age and older [ see Clinical ...
-
2 DOSAGE AND ADMINISTRATIONFor topical use only, not for oral, ophthalmic or intravaginal use [ see Clinical Studies ( 14) ]. After shaking the can well, a small amount of topical foam (e.g. a cherry-sized ...
-
3 DOSAGE FORMS AND STRENGTHSTopical foam, 4% Each gram of AMZEEQ contains 40 mg of minocycline equivalent to 43 mg of minocycline hydrochloride and is supplied as a yellow suspension in a pressurized aluminum aerosol ...
-
4 CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines or any other ingredients within AMZEEQ.
-
5 WARNINGS AND PRECAUTIONS5.1 Flammability - The propellant in AMZEEQ is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. Do not puncture and/or incinerate ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Anticoagulants - Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data with AMZEEQ use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse ...
-
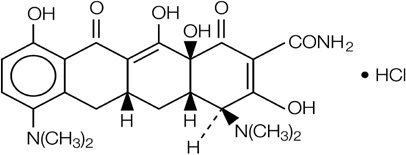
11 DESCRIPTIONMinocycline hydrochloride, a semi-synthetic derivative of tetracycline, is [4S‑ (4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of AMZEEQ for the treatment of acne is unknown. 12.2 Pharmacodynamics - The pharmacodynamics of AMZEEQ for the treatment of acne are ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a carcinogenicity study in which minocycline hydrochloride was orally administered to male and female rats once daily for up to 104 ...
-
14 CLINICAL STUDIESThe safety and efficacy of AMZEEQ was assessed in three 12-week, multicenter, randomized, double-blind, vehicle-controlled studies (Study 1 [NCT02815267], Study 2 [NCT02815280], and Study 3 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - AMZEEQ - ®(minocycline) topical foam, 4% is a yellow suspension supplied in a pressurized aluminum aerosol container (can). Each gram of AMZEEQ contains 40 mg of minocycline ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Inform patients using AMZEEQ (minocycline) topical foam, 4% of the following ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - AMZEEQ - ®(am-Zeek) (minocycline) topical foam - Important Information: AMZEEQ is for use on skin only (topical use). AMZEEQ is not ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - 30g Trade (69489-201-30)AMZEEQ Trade Label - NDC 69489- 201-30 - Label - amzeeq - ® (minocycline) topical foam, 4% For topical use only, not for oral, ophthalmic or intravaginal use - Rx only - [logo ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL - 3 g Sample (69489-201-03)AMZEEQ Sample Labels - NDC 69489- 201-03 - Label - Physician Sample Not for Sale - amzeeq - ® (minocycline) topical foam, 4% For topical use only, not for oral, ophthalmic or ...
-
INGREDIENTS AND APPEARANCEProduct Information