Label: AMYVID- florbetapir f 18 injection, solution
- NDC Code(s): 0002-1200-48, 0002-1200-50
- Packager: Eli Lilly and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMYVID safely and effectively. See full prescribing information for AMYVID. AMYVID (Florbetapir F 18 Injection) for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Amyvid is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety-Drug Handling - Amyvid is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration [see Warnings and ...
-
3 DOSAGE FORMS AND STRENGTHS
Amyvid (Florbetapir F 18 Injection) is available in a 50 mL and 100 mL multidose vial containing a clear, colorless solution at a strength of 500-1900 MBq/mL (13.5-51 mCi/mL) florbetapir F 18 at ...
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
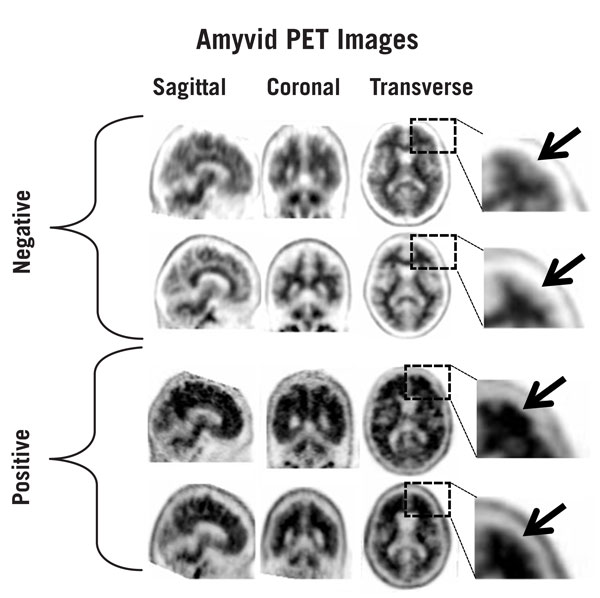
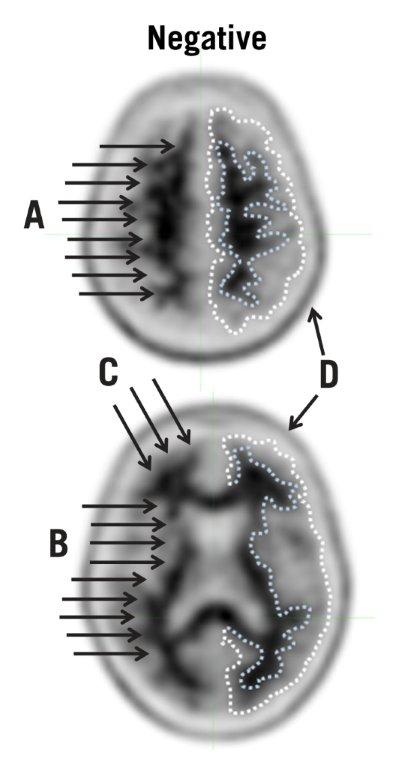
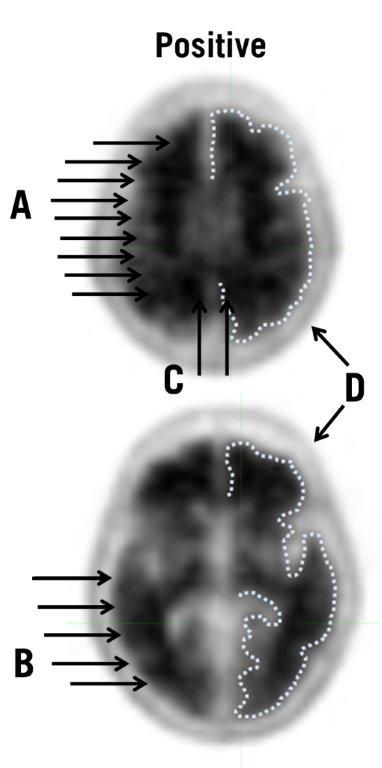
5.1 Risk for Image Misinterpretation and other Errors - Errors may occur in the Amyvid estimation of brain neuritic plaque density during image interpretation [see Clinical Studies (14)]. Image ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
Pharmacodynamic drug-drug interaction studies have not been performed in patients to establish the extent, if any, to which concomitant medications may alter Amyvid image results. Within a ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are no available data on Amyvid use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or ...
-
11 DESCRIPTION
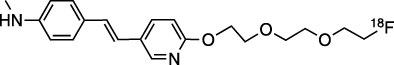
Amyvid contains florbetapir F 18, a molecular imaging agent that binds to β-amyloid aggregates, and is intended for use with PET imaging of the brain. Chemically, florbetapir F 18 is described as ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Florbetapir F 18 binds to β-amyloid plaques and the F 18 isotope produces a positron signal that is detected by a PET scanner. In in vitro binding studies using ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies to assess the carcinogenicity or reproductive toxicity potentials of Amyvid have not been conducted. In an in vitro ...
-
14 CLINICAL STUDIES
Amyvid was evaluated in three clinical studies that examined images from healthy adult subjects as well as subjects with a range of cognitive disorders, including some terminally ill patients who ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - Amyvid is supplied in 50 mL or 100 mL vials containing 10-50 mL or 10-100 mL respectively, of a clear, colorless solution at a strength of 500 - 1900 MBq/mL ...
-
17 PATIENT COUNSELING INFORMATION
Instruct patients to inform their physician or healthcare provider if they are pregnant or breastfeeding. Inform patients who are breastfeeding to use alternate infant nutrition sources (e.g. ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – Amyvid 50 mL PETNET Label - NDC Code 0002-1200-50 50 mL Multiple-Dose Vial - Sterile - Rx only - ☢ CAUTION: RADIOACTIVE MATERIAL - AmyvidTM Florbetapir F 18 ...
-
INGREDIENTS AND APPEARANCEProduct Information