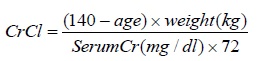Label: AMPYRA- dalfampridine tablet, film coated, extended release
- NDC Code(s): 10144-427-60
- Packager: Merz Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMPYRA safely and effectively. See full prescribing information for AMPYRA. AMPYRA ® (dalfampridine) extended-release tablets ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAMPYRA is indicated as a treatment to improve walking in adult patients with multiple sclerosis (MS). This was demonstrated by an increase in walking speed [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage Information - The maximum recommended dosage of AMPYRA is one 10 mg tablet twice daily and should not be exceeded. Take doses approximately 12 hours apart. There is no evidence of ...
-
3 DOSAGE FORMS AND STRENGTHSAMPYRA is available in a 10 mg strength and is a film-coated, white to off-white, biconvex, oval shaped, non-scored tablet with flat edge, debossed with "A10" on one side.
-
4 CONTRAINDICATIONSThe use of AMPYRA is contraindicated in the following conditions: History of seizure [see Warnings and Precautions (5.1)] Moderate or severe renal impairment (CrCl≤50 mL/min) [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Seizures - AMPYRA can cause seizures. Increased incidence of seizures has been observed at 20 mg twice daily (2 times the maximum recommended dosage) in controlled clinical studies of 9–14 ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described in more detail elsewhere in the labeling: Seizures [see Warnings and Precautions (5.1)] Anaphylaxis [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 OCT2 Inhibitors - Concurrent treatment with OCT2 inhibitors, such as cimetidine, may cause increased exposure to dalfampridine [see Clinical Pharmacology (12.3)]. Elevated levels of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with use of AMPYRA in pregnant women. Administration of dalfampridine to animals during pregnancy ...
-
10 OVERDOSAGEThree cases of overdose were reported in controlled clinical trials with AMPYRA, involving two MS patients. The first patient took six times the currently recommended dose (60 mg) and was taken to ...
-
11 DESCRIPTIONAMPYRA (dalfampridine) is a potassium channel blocker, available in a 10 mg tablet strength. Each tablet contains 10 mg dalfampridine, formulated as an extended-release tablet for twice-daily oral ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of action - The mechanism by which dalfampridine exerts its therapeutic effect has not been fully elucidated. Dalfampridine is a broad spectrum potassium channel blocker. In animal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Two-year dietary carcinogenicity studies of dalfampridine were conducted in mice and rats. In mice, the doses tested ...
-
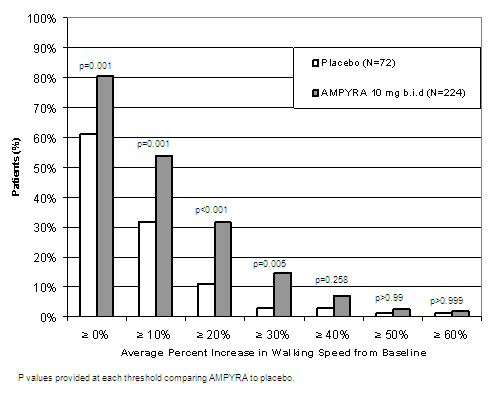
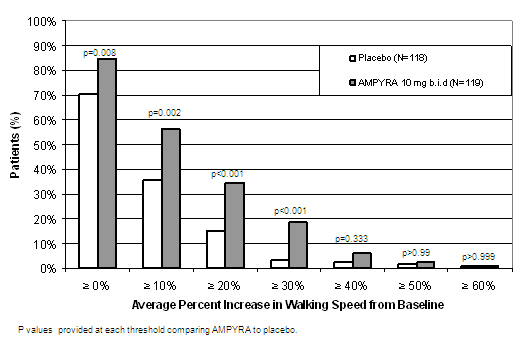
14 CLINICAL STUDIESThe effectiveness of AMPYRA in improving walking in patients with multiple sclerosis was evaluated in two adequate and well controlled trials involving 540 patients. Patients in these two clinical ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAMPYRA (dalfampridine) extended release tablets, 10 mg are film-coated, white to off-white, biconvex, oval shaped, non-scored tablets with flat edge. The tablets are identified by a debossed code ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Risk of Seizures - Inform patients that AMPYRA can cause seizures, and that they must discontinue use of AMPYRA ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Acorda Therapeutics, Inc. Pearl River, NY 10965 - AMPYRA® is a registered trademark of Acorda Therapeutics, Inc. ©2022 Acorda Therapeutics, Inc. All rights reserved. 2212427AK-1
-
MEDICATION GUIDEMEDICATION GUIDE - AMPYRA® (am-PEER-ah) (dalfampridine) Extended Release Tablets - Read this Medication Guide before you start taking AMPYRA and each time you get a refill. There may be new ...
-
SPL UNCLASSIFIED SECTIONAMPYRA® is a registered trademark of Acorda Therapeutics, Inc. ©2022 Acorda Therapeutics, Inc. All rights reserved. 2212427AK-1
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 10 mg Tablet Label - ampyra® (dalfampridine) Extended Release - Tablets - Rx ONLY - 10 mg - PHARMACIST: Dispense the accompanying - Medication Guide to each patient ...
-
INGREDIENTS AND APPEARANCEProduct Information