Label: GLIMEPIRIDE tablet
- NDC Code(s): 68001-177-00, 68001-177-03, 68001-178-00, 68001-178-03, view more
- Packager: BluePoint Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GLIMEPIRIDE TABLETS safely and effectively. See full prescribing information for GLIMEPIRIDE TABLETS. GLIMEPIRIDE Tablets ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEGlimepiride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Glimepiride tablets should be administered with breakfast or the first main meal of the day. The recommended starting dose of glimepiride tablet is 1 mg or 2 mg once ...
-
3 DOSAGE FORMS AND STRENGTHSGlimepiride tablets, USP are formulated as tablets of: 1 mg tablets (pink coloured, oval shaped, biconvex, uncoated tablets debossed with ‘AHI 1’ on one side and break line on the other ...
-
4 CONTRAINDICATIONSGlimepiride tablet is contraindicated in patients with a history of a hypersensitivity reaction to: Glimepiride or any of the product’s ingredients [see Warnings ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypoglycemia - All sulfonylureas, including glimepiride, can cause severe hypoglycemia [see Adverse Reactions ( 6.1)]. The ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed in more detail below and elsewhere in the labeling: Hypoglycemia [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Drugs Affecting Glucose Metabolism - A number of medications affect glucose metabolism and may require glimepiride dose adjustment and particularly close monitoring for hypoglycemia or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from a small number of published studies and postmarketing experience with glimepiride use in pregnancy over decades have not identified any drug ...
-
10 OVERDOSAGEAn overdosage of glimepiride, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions constitute ...
-
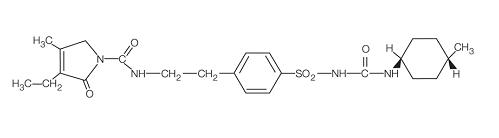
11 DESCRIPTIONGlimepiride is an oral sulfonylurea that contains the active ingredient glimepiride. Chemically, glimepiride is identified as 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1‑carboxamido ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Glimepiride primarily lowers blood glucose by stimulating the release of insulin from pancreatic beta cells. Sulfonylureas bind to the sulfonylurea receptor in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Studies in rats at doses of up to 5000 parts per million (ppm) in complete feed (approximately 340 times the maximum recommended ...
-
14 CLINICAL STUDIES14.1 Monotherapy - A total of 304 patients with type 2 diabetes already treated with sulfonylurea therapy participated in a 14-week, multicenter, randomized, double-blind, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGGlimepiride tablets USP are available in the following strengths and package sizes: 1 mg tablets: pink coloured, oval shaped, biconvex, uncoated tablets debossed with ‘AHI 1’ on one side and ...
-
17 PATIENT COUNSELING INFORMATION17.1 Information for Patients - Hypoglycemia - Explain the symptoms and treatment of hypoglycemia as well as conditions that predispose to hypoglycemia. Inform patients that their ability to ...
-
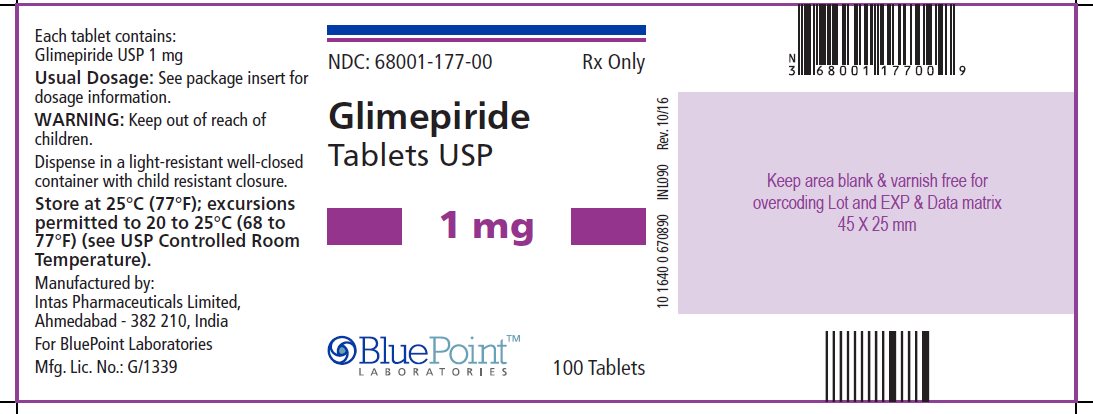
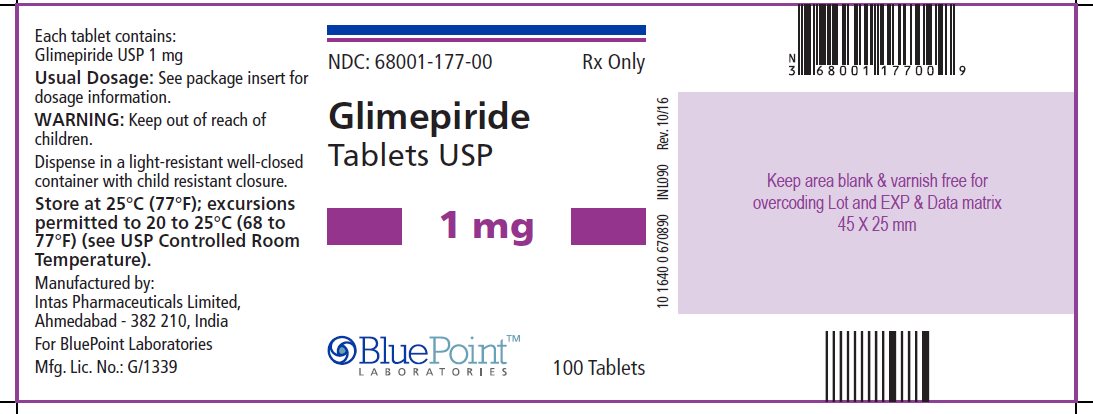
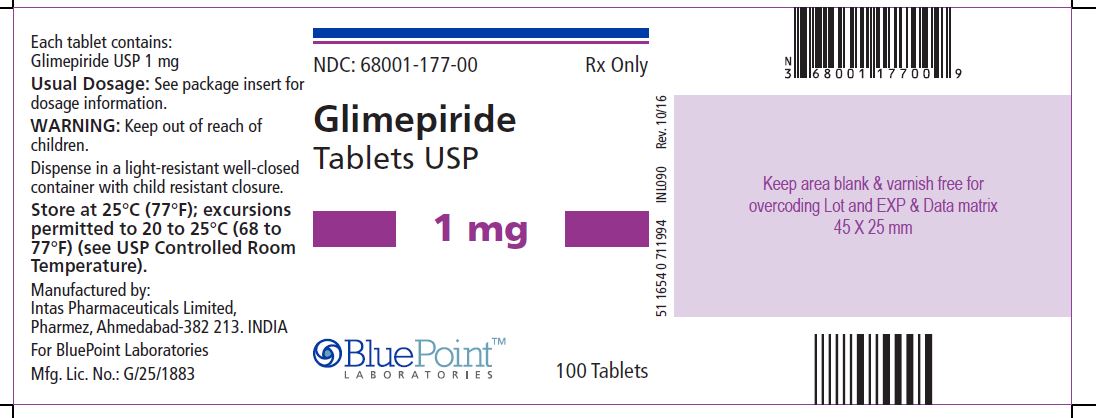
Package/Label Display PanelGlimepiride Tablets USP, 1 mg, 100 Tablets - NDC 68001-177-00 - Manufactured by: Intas Pharmaceuticals Limited, Ahmedabad – 382 210, India, For BluePoint Laboratories, Mfg. Lic. No.: G/1339
-
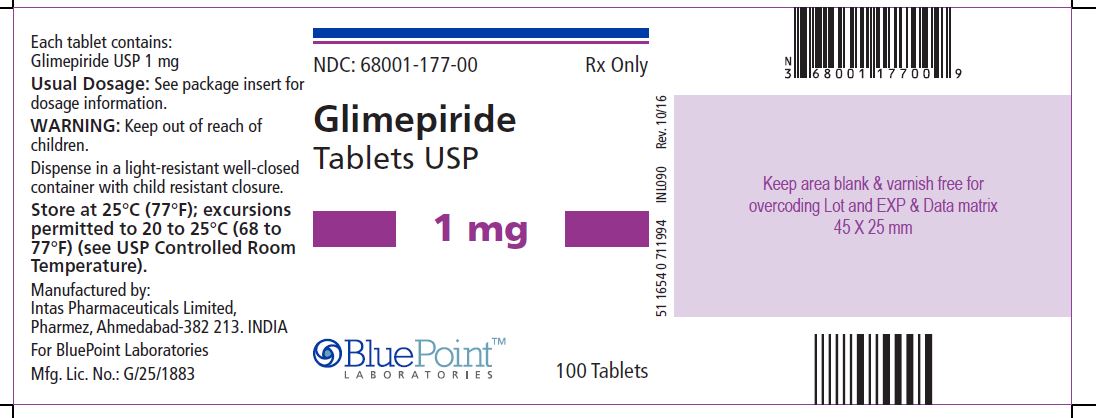
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Glimepiride Tablets USP, 1 mg, 100 Tablets - NDC 68001-177-00 - Manufactured by: Intas Pharmaceuticals Limited, Ahmedabad – 382 213, India, For BluePoint Laboratories, Mfg. Lic. No. ...
-
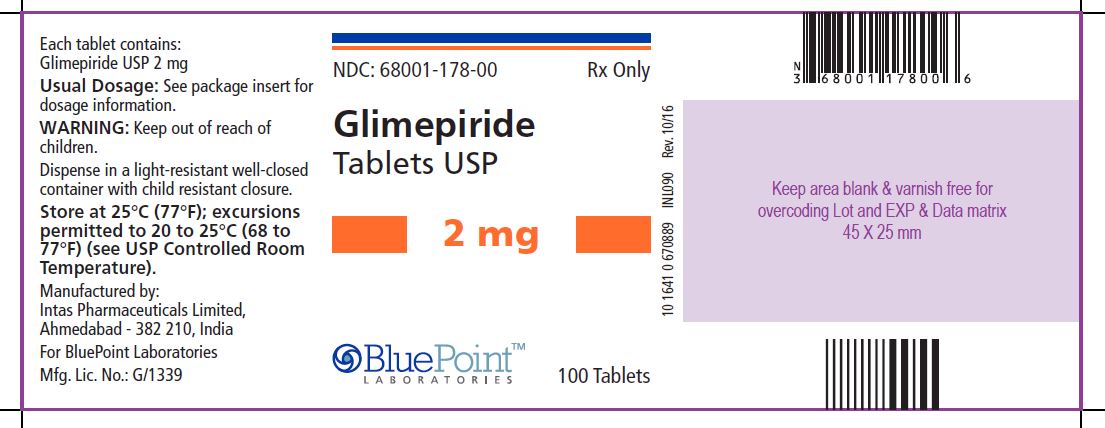
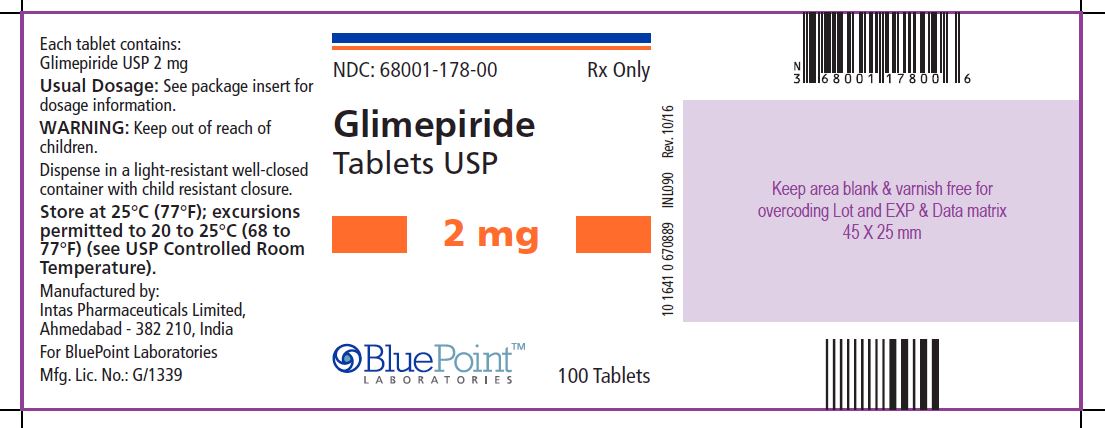
Package/Label Display PanelGlimepiride Tablets USP, 2 mg, 100 Tablets - NDC 68001-178-00 - Manufactured by: Intas Pharmaceuticals Limited, Ahmedabad – 382 210, India, For BluePoint Laboratories, Mfg. Lic. No.: G/1339
-
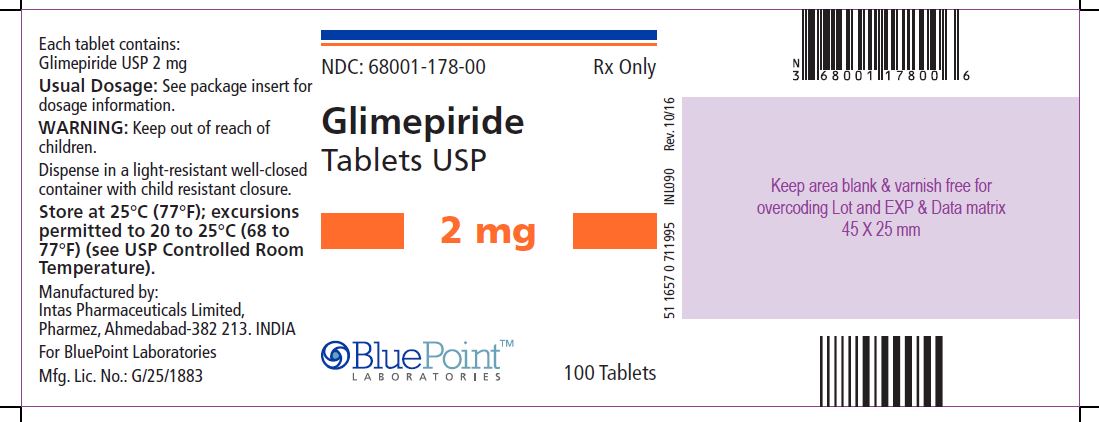
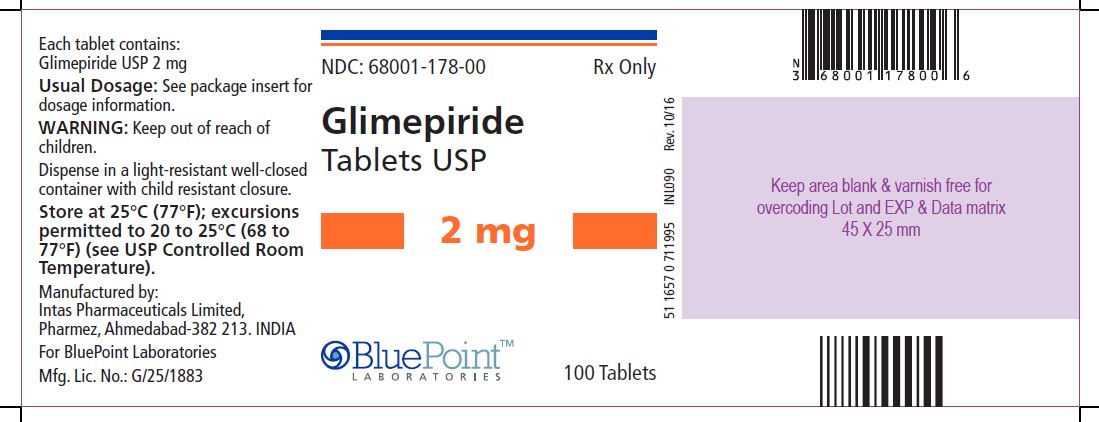
Package/Label Display PanelGlimepiride Tablets USP, 2 mg, 100 Tablets - NDC 68001-178-00 - Manufactured by: Intas Pharmaceuticals Limited, Pharmez, Ahmedabad – 382 213, India, For BluePoint Laboratories, Mfg. Lic. No. ...
-
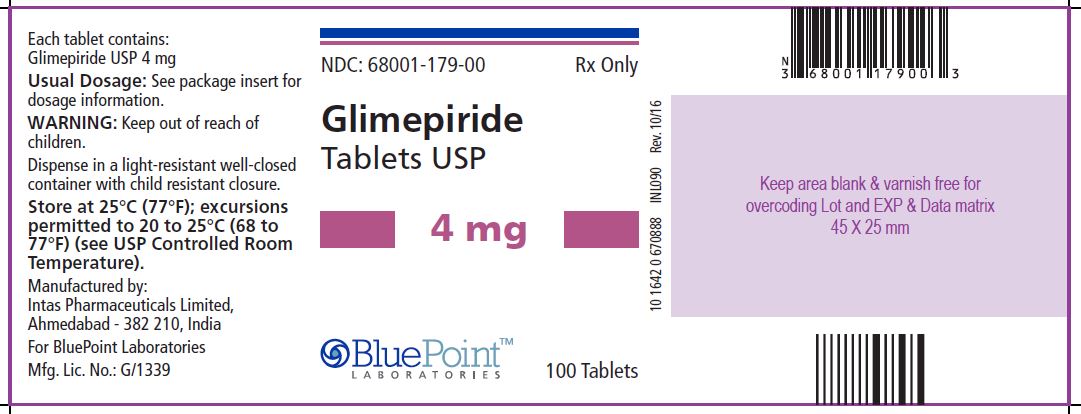
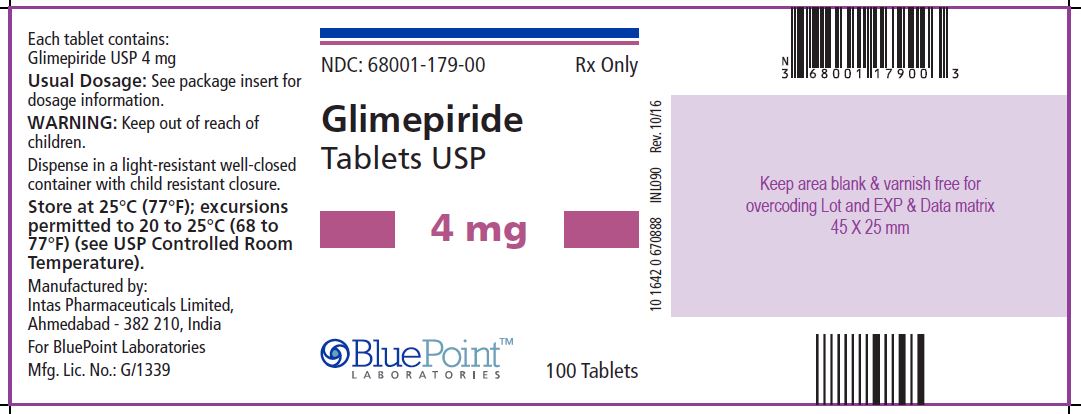
Package/Label Display Panel Glimepiride Tablets USP, 4 mg, 100 Tablets - NDC 68001-179-00 - Manufactured by: Intas Pharmaceuticals Limited, Ahmedabad – 382 210, India, For BluePoint Laboratories, Mfg. Lic. No.: G/1339
-
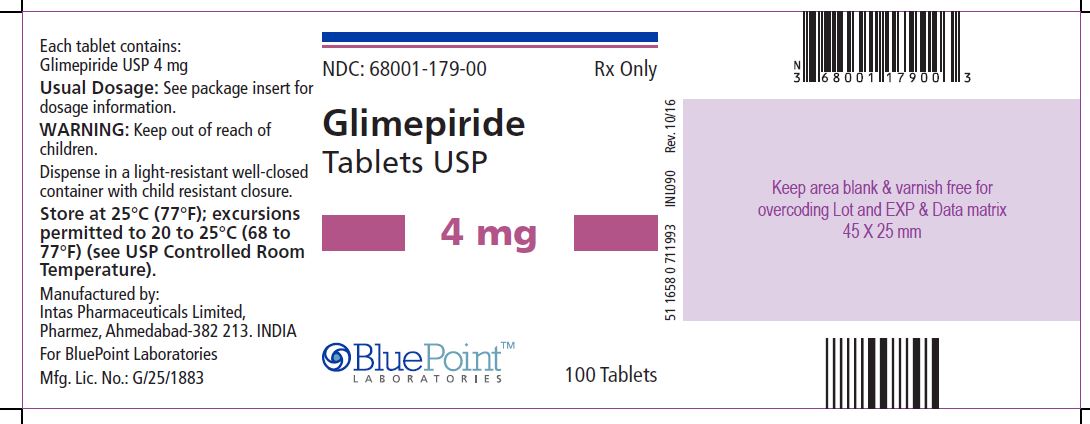
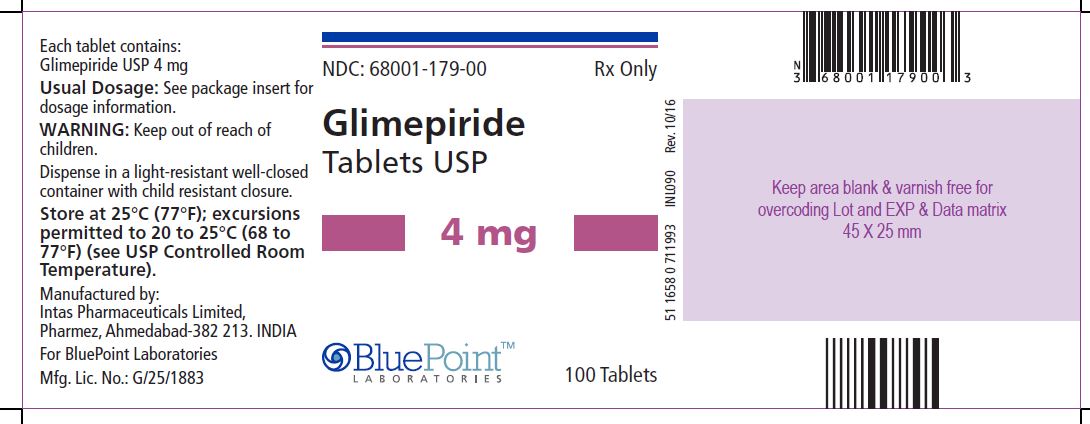
Package/Label Display Panel Glimepiride Tablets USP, 4 mg, 100 Tablets - NDC 68001-179-00 - Manufactured by: Intas Pharmaceuticals Limited, Pharmez, Ahmedabad – 382 213, India, For BluePoint Laboratories, Mfg. Lic. No. ...
-
INGREDIENTS AND APPEARANCEProduct Information