Label: ALTRENO- tretinoin lotion
- NDC Code(s): 0187-0005-03, 0187-0005-04, 0187-0005-10, 0187-0005-20, view more
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 2, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ALTRENO safely and effectively. See full prescribing information for ALTRENO. ALTRENO® (tretinoin) lotion, for topical use - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE ALTRENO® (tretinoin) lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
-
2 DOSAGE AND ADMINISTRATION Apply a thin layer of ALTRENO to the affected areas once daily. Avoid the eyes, mouth, paranasal creases, and mucous membranes. ALTRENO is for topical use only. Not for ophthalmic, oral, or ...
-
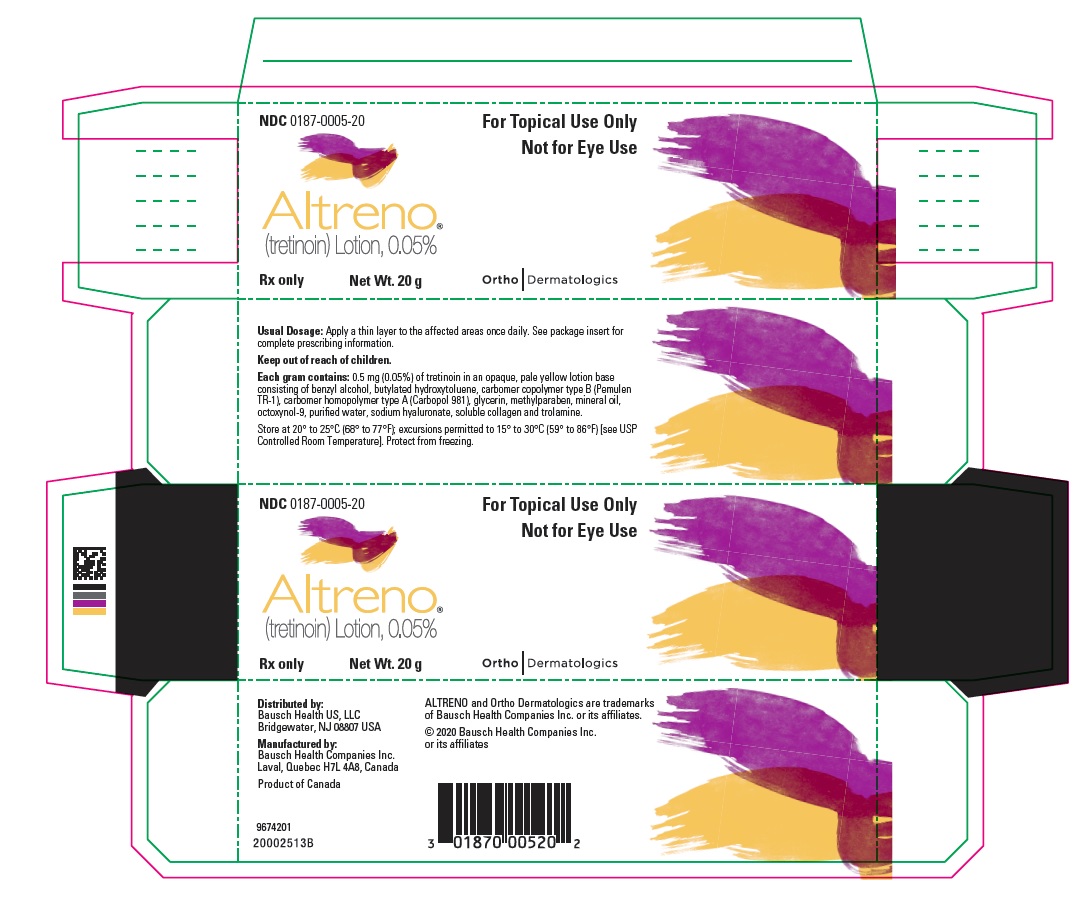
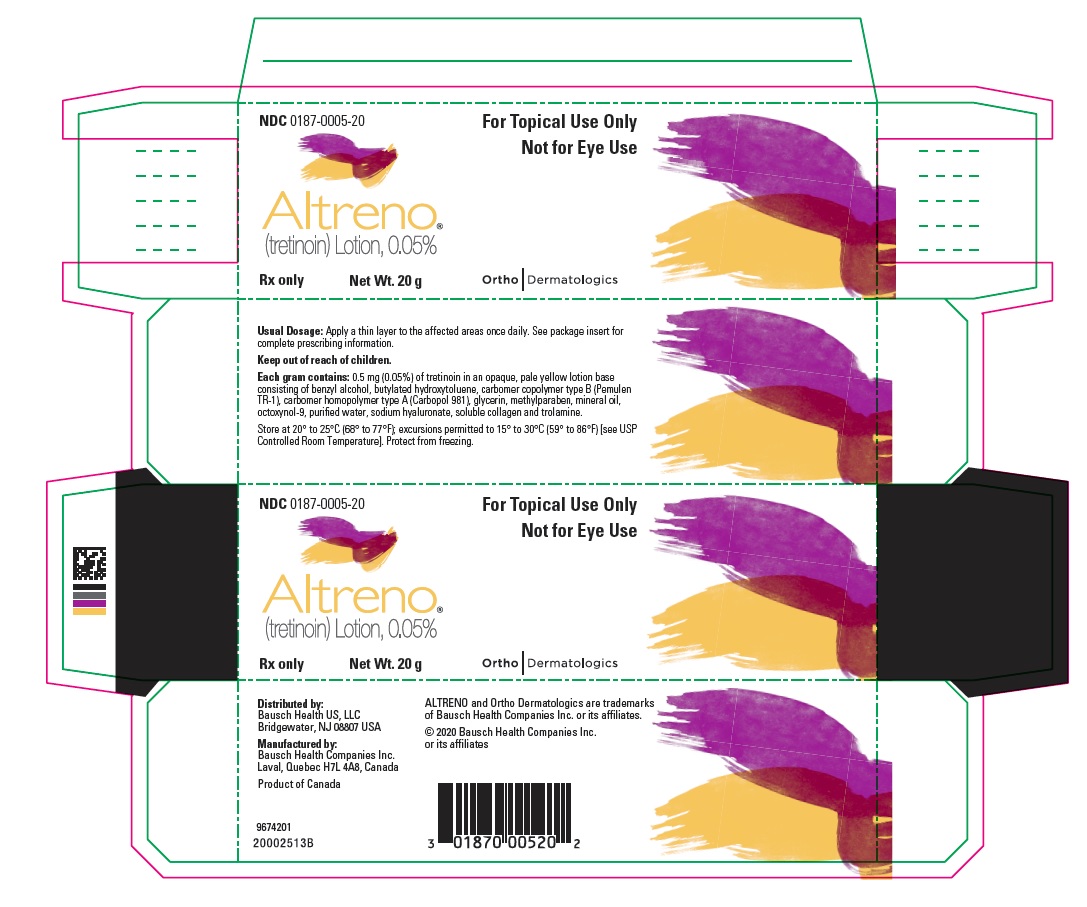
3 DOSAGE FORMS AND STRENGTHS Lotion, 0.05% Each gram of ALTRENO contains 0.5 mg (0.05%) tretinoin in an opaque, pale yellow topical lotion.
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Skin Irritation Patients using ALTRENO may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data from published observational studies of topical tretinoin in pregnant women have not established a drug-associated risk of major birth defects ...
-
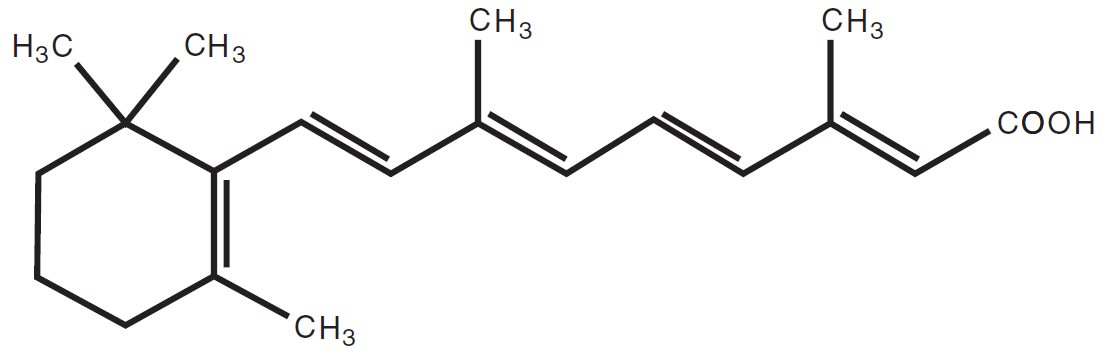
11 DESCRIPTION ALTRENO (tretinoin) lotion is an opaque, pale yellow lotion containing 0.05% tretinoin by weight for topical administration. Chemically, tretinoin is all-trans-retinoic acid, also known as ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tretinoin is a metabolite of vitamin A that binds with high affinity to specific retinoic acid receptors located in both the cytosol and nucleus. Tretinoin activates ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 2-year dermal mouse carcinogenicity study was conducted with topical administration of 0.005%, 0.025% and 0.05% of a tretinoin gel ...
-
14 CLINICAL STUDIES The safety and efficacy of once daily use of ALTRENO for the treatment of acne vulgaris were assessed in two multicenter, randomized, double-blind clinical trials enrolling 1640 subjects age 9 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING ALTRENO (tretinoin) lotion, 0.05% is an opaque, pale yellow topical lotion and available as: • 45 g tube (NDC 0187-0005-45) • 20 g tube (NDC 0187-0005-20) • 50 g pump (NDC ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). • Warn patients of the potential for skin irritation during treatment. • Advise patients to minimize exposure ...
-
Patient Package Insert PATIENT INFORMATION - ALTRENO® (al-TREN-oh) (tretinoin) lotion, 0.05% for topical use - Important information: ALTRENO is for use on skin only. Do not use ALTRENO in your eyes, mouth, the corners of ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Carton 20 gNDC 0187-0005-20 - For Topical Use Only - Not for Eye Use - ALTRENO® (tretinoin) Lotion, 0.05% Rx only - Net Wt. 20 g - Ortho Dermatologics
-
INGREDIENTS AND APPEARANCEProduct Information