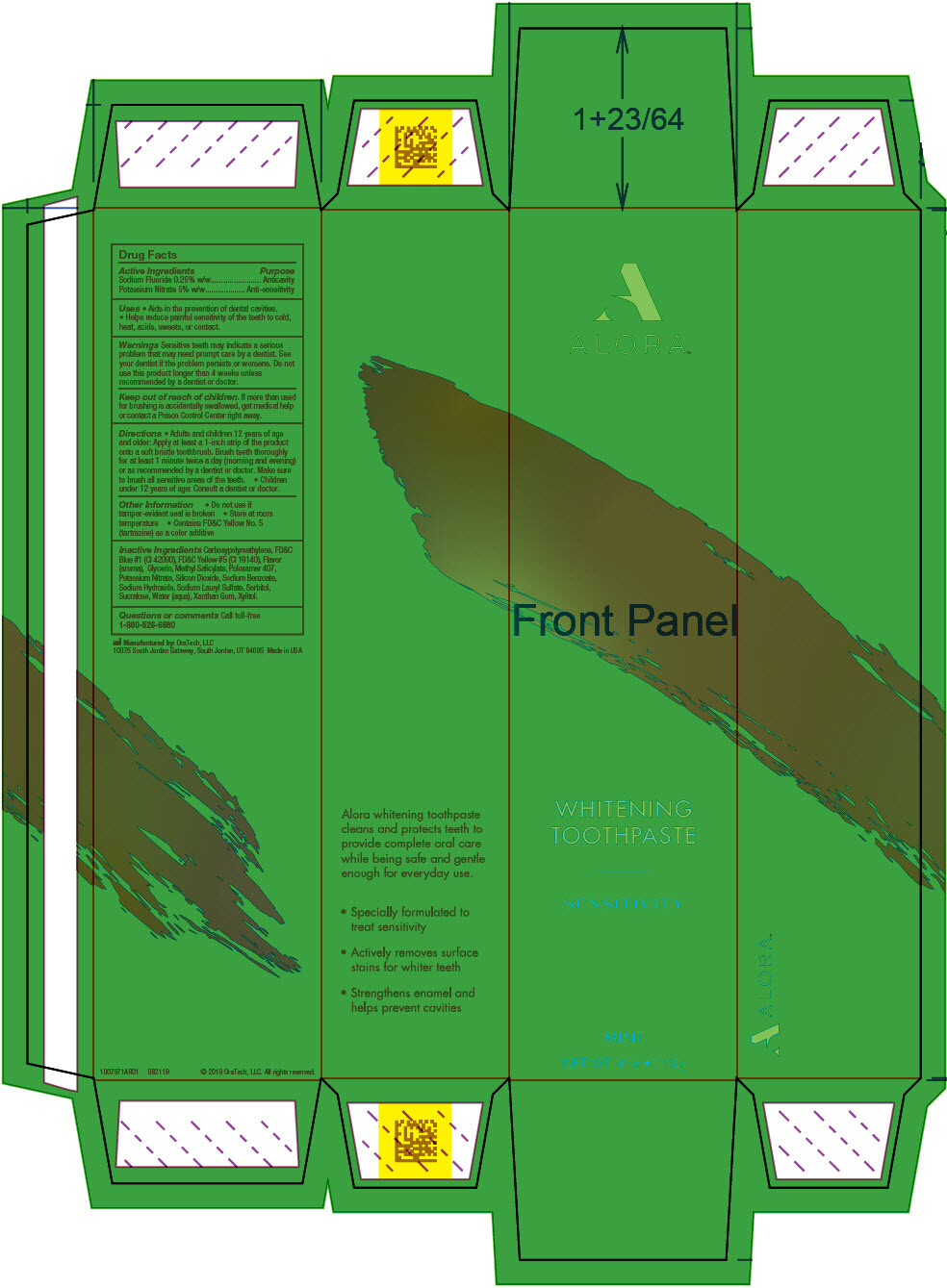
Label: ALORA SENSITIVITY- potassium nitrate and sodium fluoride gel, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 72288-309-01, 72288-309-02, 72288-309-03 - Packager: Amazon, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
ACTIVE INGREDIENTActive IngredientsPurpose - Sodium Fluoride 0.25% w/wAnticavity - Potassium Nitrate 5% w/wAnti-sensitivity
-
UsesAids in the prevention of dental cavities. Helps reduce painful sensitivity of the teeth to cold, heat, acids, sweets, or contact.
-
WarningsSensitive teeth may indicate a serious problem that may need prompt care by a dentist. See your dentist if the problem persists or worsens. Do not use this product longer than 4 weeks unless ...
-
DirectionsAdults and children 12 years of age and older: Apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and ...
-
Other InformationDo not use if tamper-evident seal is broken - Store at room temperature - Contains FD&C Yellow No. 5 (tartrazine) as a color additive
-
Inactive IngredientsCarboxypolymethylene, FD&C Blue #1 (CI 42090), FD&C Yellow #5 (CI 19140), Flavor (aroma), Glycerin, Methyl Salicylate, Poloxamer 407, Potassium Nitrate, Silicon Dioxide, Sodium Benzoate, Sodium ...
-
Questions or commentsCall toll-free 1-800-526-6880
-
SPL UNCLASSIFIED SECTIONManufactured by: OraTech, LLC - 10075 South Jordan Gateway, South Jordan, UT 84095
-
PRINCIPAL DISPLAY PANEL - 113 g Tube CartonALORA™ WHITENING - TOOTHPASTE - SENSITIVITY - MINT - NET WT. 4 oz • 113 g
-
INGREDIENTS AND APPEARANCEProduct Information