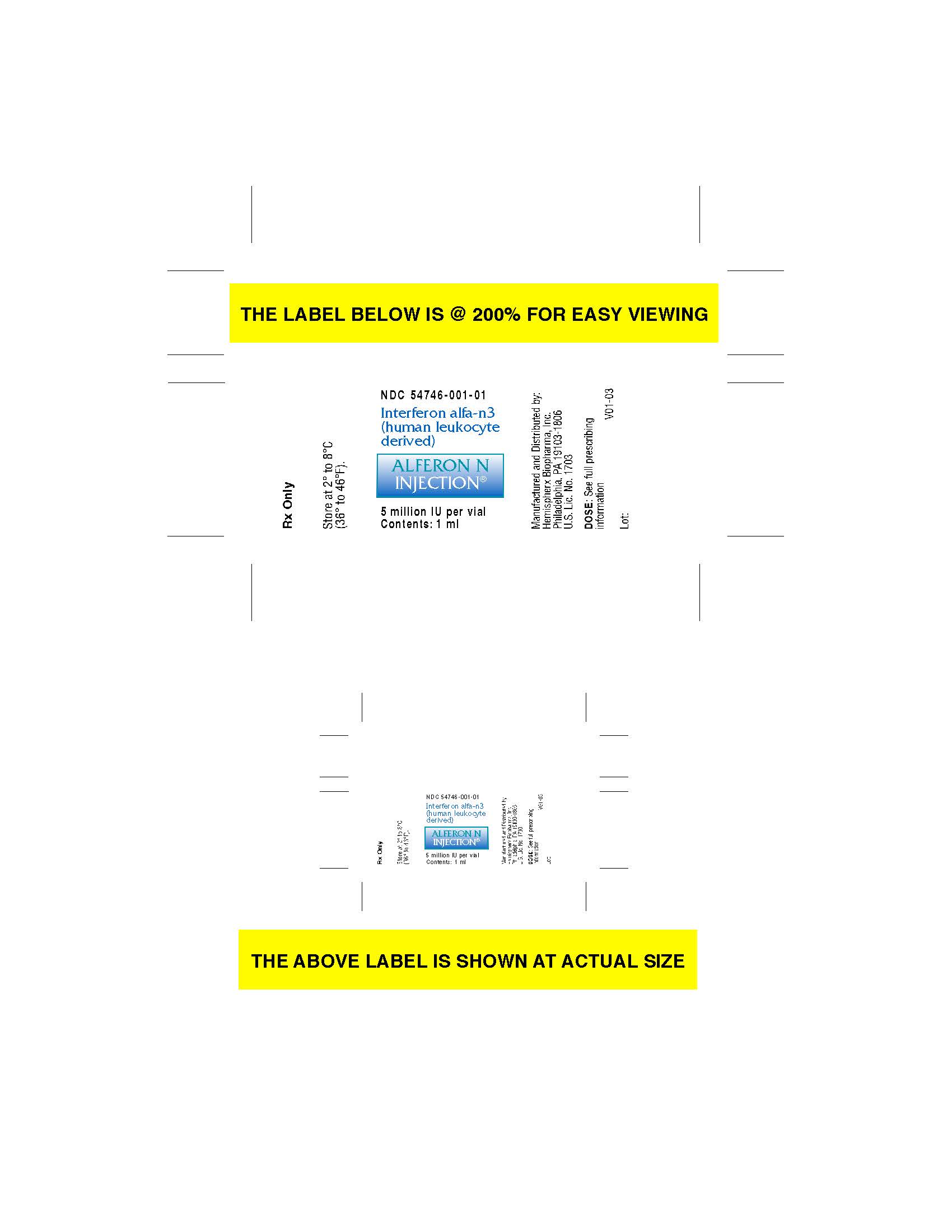
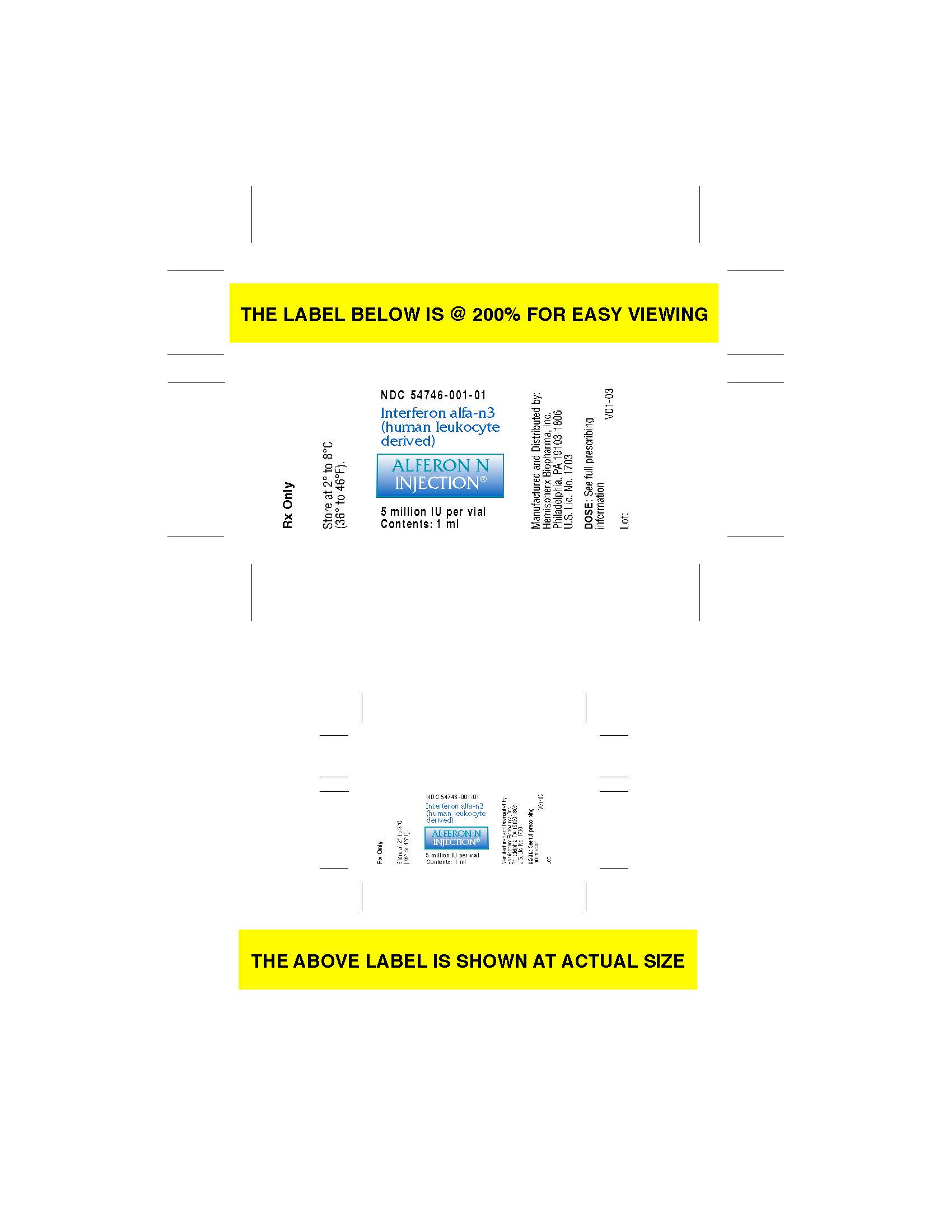
Label: ALFERON- interferon alfa-n3 injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 54746-001-01 - Packager: AIM ImmunoTech Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 28, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
StorageSTORAGE Alferon N Injection® [Interferon alfa-n3 (human leukocyte derived)] should be stored at 2º to 8ºC (36º to 46ºF). Do not Freeze. Do not shake.
-
carton label

-
vial label
-
INGREDIENTS AND APPEARANCEProduct Information