Label: AIMOVIG- erenumab-aooe injection
AIMOVIG- erenumab-aooe injection, solution
- NDC Code(s): 55513-840-01, 55513-840-02, 55513-841-00, 55513-841-01, view more
- Packager: Amgen Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AIMOVIG safely and effectively. See full prescribing information for AIMOVIG. AIMOVIG® (erenumab-aooe) injection, for subcutaneous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAIMOVIG is indicated for the preventive treatment of migraine in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - The recommended dosage of AIMOVIG is 70 mg injected subcutaneously once monthly. Some patients may benefit from a dosage of 140 mg injected subcutaneously once ...
-
3 DOSAGE FORMS AND STRENGTHSAIMOVIG is a sterile, clear to opalescent, colorless to light yellow solution available as follows: Injection: 70 mg/mL in a single-dose prefilled SureClick® autoinjector - Injection: 140 mg/mL ...
-
4 CONTRAINDICATIONSAIMOVIG is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with AIMOVIG in postmarketing experience. Most hypersensitivity ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Constipation with Serious Complications ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AIMOVIG during pregnancy. Patients should be ...
-
11 DESCRIPTIONErenumab-aooe is a human immunoglobulin G2 (IgG2) monoclonal antibody that has high affinity binding to the calcitonin gene-related peptide receptor. Erenumab-aooe is produced using recombinant ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Erenumab-aooe is a human monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) receptor and antagonizes CGRP receptor function. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of erenumab-aooe has not been assessed. Mutagenesis - Genetic toxicology studies of ...
-
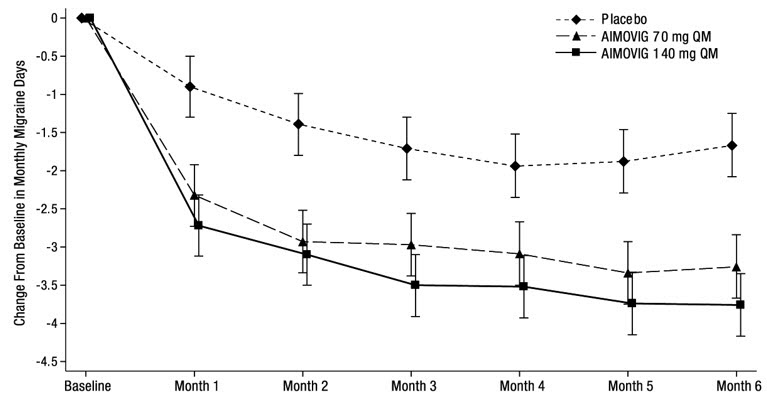
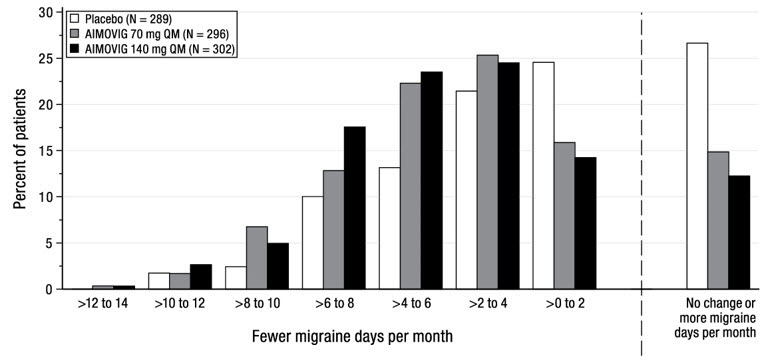
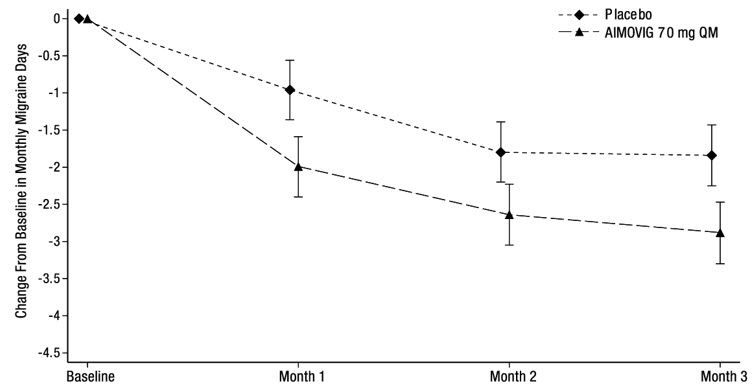
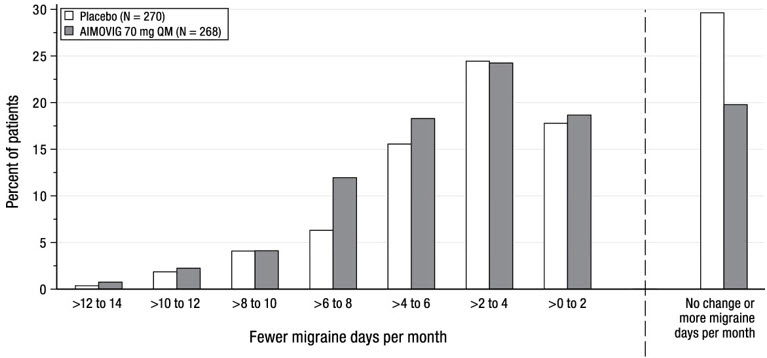
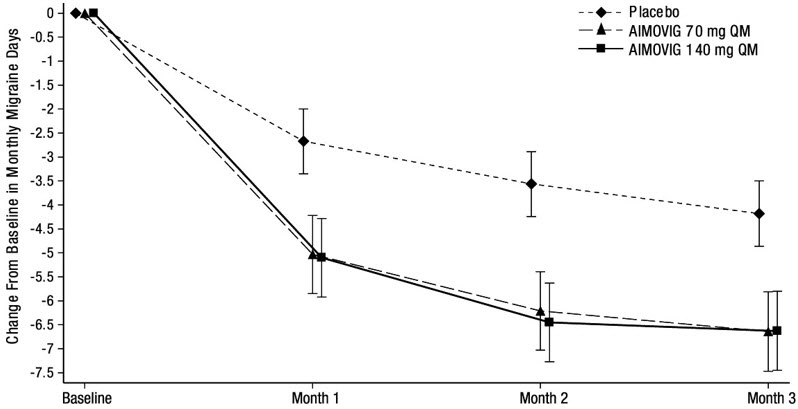
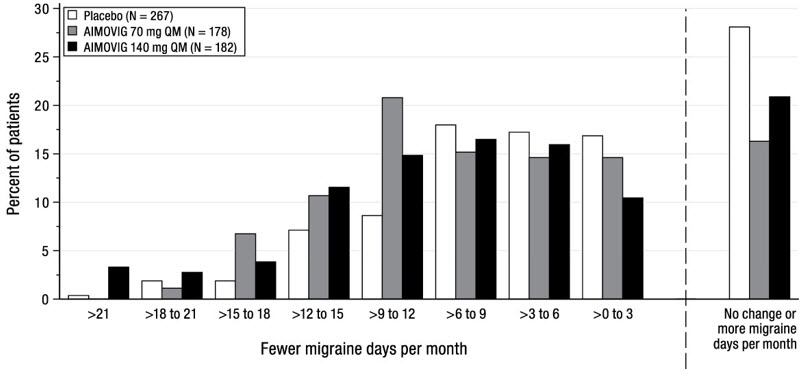
14 CLINICAL STUDIESThe efficacy of AIMOVIG was evaluated as a preventive treatment of episodic or chronic migraine in three randomized, double-blind, placebo-controlled studies: two studies in patients with episodic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - AIMOVIG (erenumab-aooe) injection is a sterile, clear to opalescent, colorless to light yellow solution for subcutaneous administration. AIMOVIG prefilled autoinjectors and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Information on Preparation and Administration: Provide guidance to patients and ...
-
SPL UNCLASSIFIED SECTIONFor more information, go to www.aimovig.com or call 1-800-77-AMGEN (1-800-772-6436). AIMOVIG® (erenumab-aooe) Manufactured by: Amgen Inc. One Amgen Center Drive - Thousand Oaks, CA 91320-1799 ...
-
PATIENT PACKAGE INSERTPatient Information - AIMOVIG® (AIM-oh-vig) (erenumab-aooe) injection, for subcutaneous use - This Patient Information has been approved by the U.S. Food and Drug Administration. [part ...
-
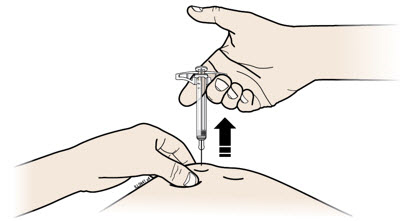
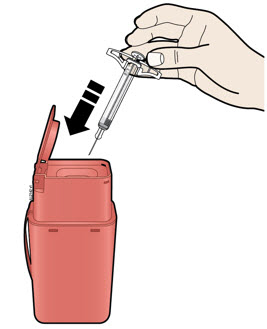
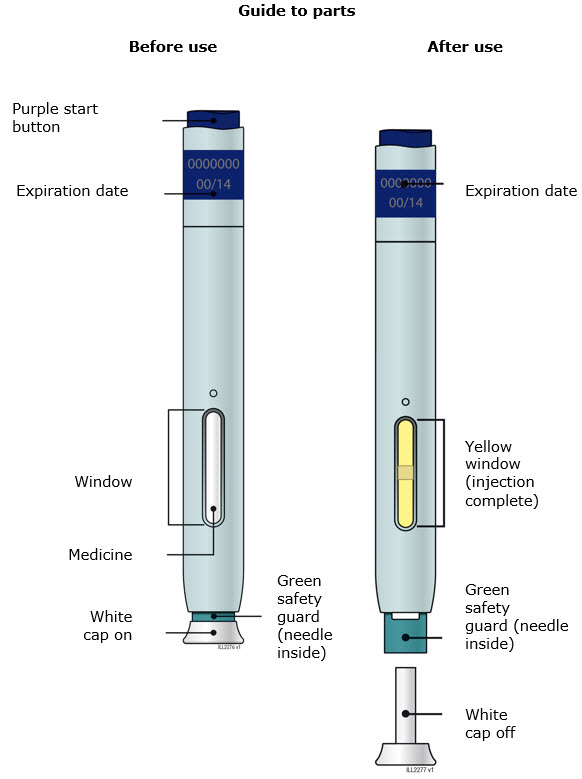
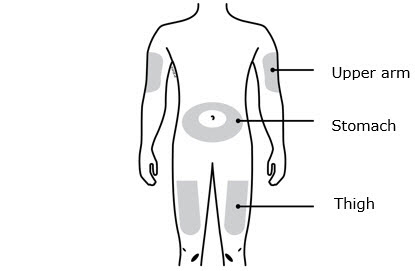
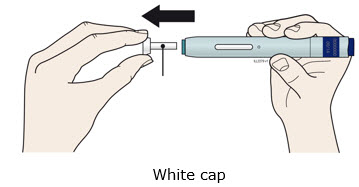
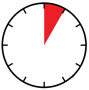
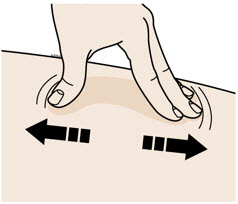
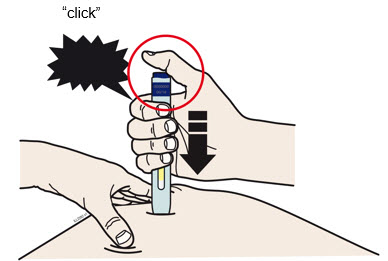
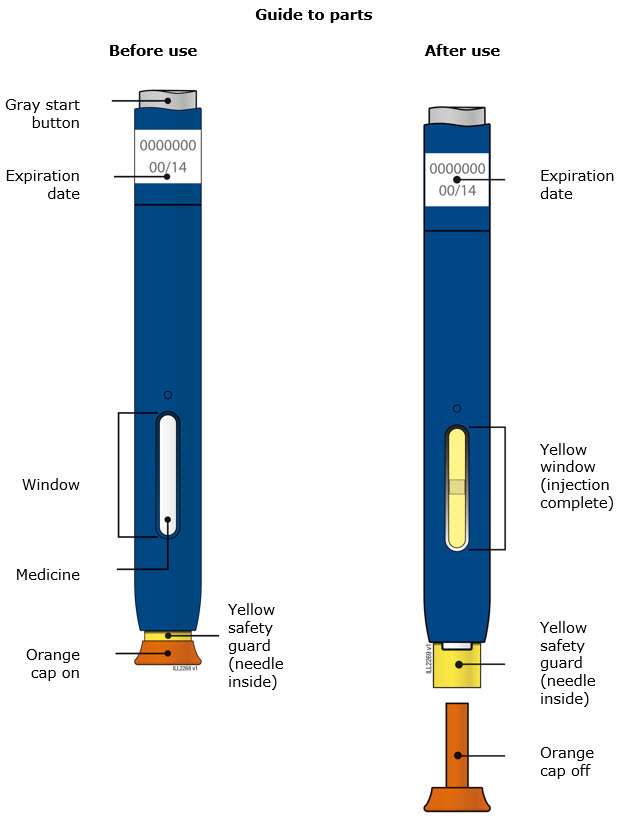
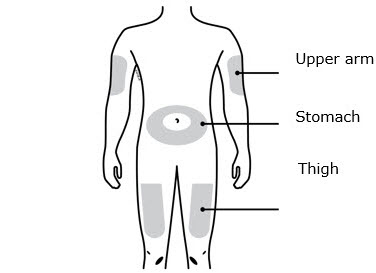
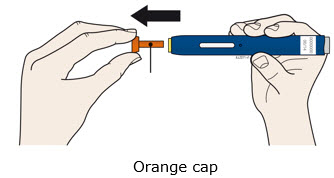
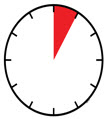
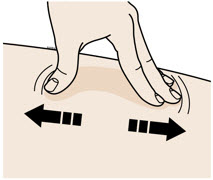
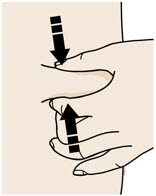
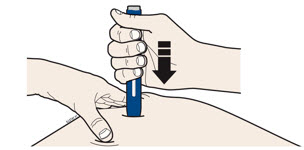
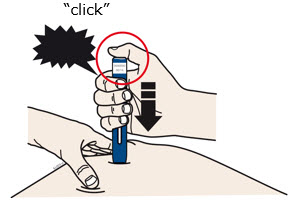
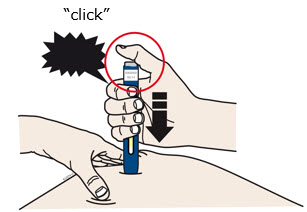
INSTRUCTIONS FOR USEAIMOVIG® (AIM-oh-vig)(erenumab-aooe)injection, for subcutaneous use70 mg/mLsingle-dose prefilled SureClick® autoinjectorThis Instructions for Use contains information on how to inject AIMOVIG with a SureClick autoinjector. If your healthcare provider decides that you or a caregiver may be able to give your ...
-
REFERENCE GUIDE Side 1Reference Guide - Aimovig® (AIM-oh-vig) (erenumab-aooe) injection, for subcutaneous use - Single-Dose Prefilled SureClick® Autoinjector - 70 mg/mL Read all instructions in carton before ...
-
INSTRUCTIONS FOR USEAIMOVIG® (AIM-oh-vig)(erenumab-aooe)injection, for subcutaneous use140 mg/mLsingle-dose prefilled SureClick® autoinjectorThis Instructions for Use contains information on how to inject AIMOVIG with a SureClick autoinjector. If your healthcare provider decides that you or a caregiver may be able to give your ...
-
REFERENCE GUIDE Side 1Reference Guide - Aimovig® (AIM-oh-vig) (erenumab-aooe) injection, for subcutaneous use - Single-Dose Prefilled SureClick® Autoinjector - 140 mg/mL Read all instructions in carton before ...
-
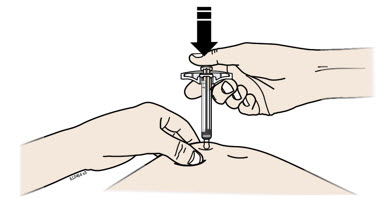
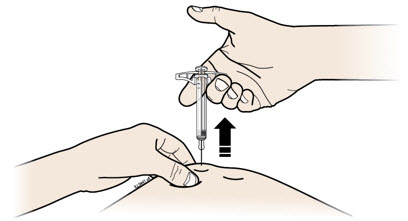
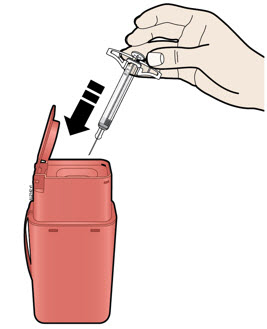
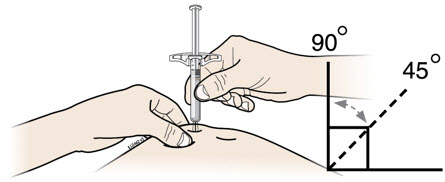
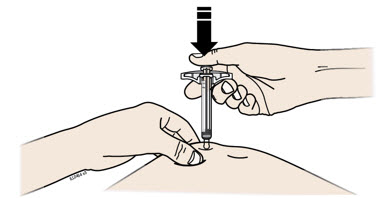
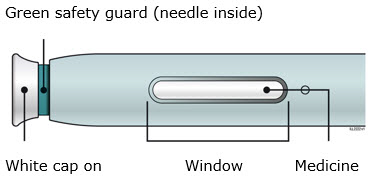
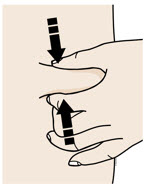
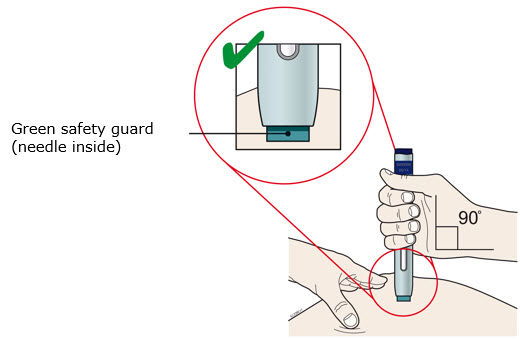
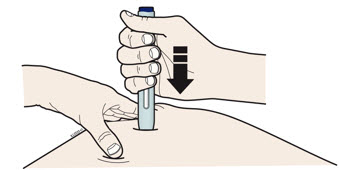
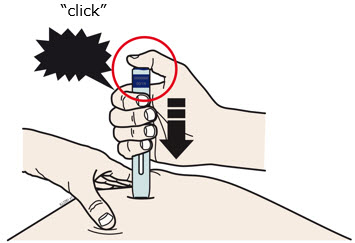
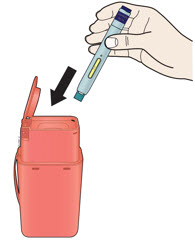
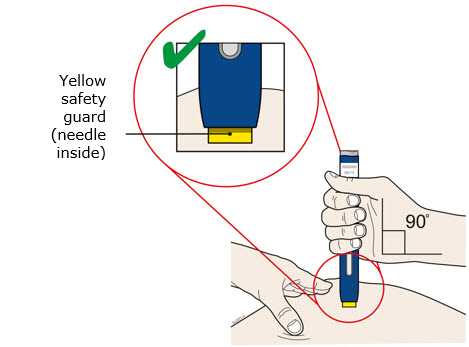
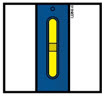
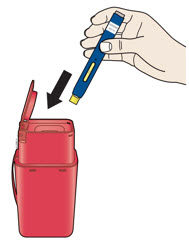
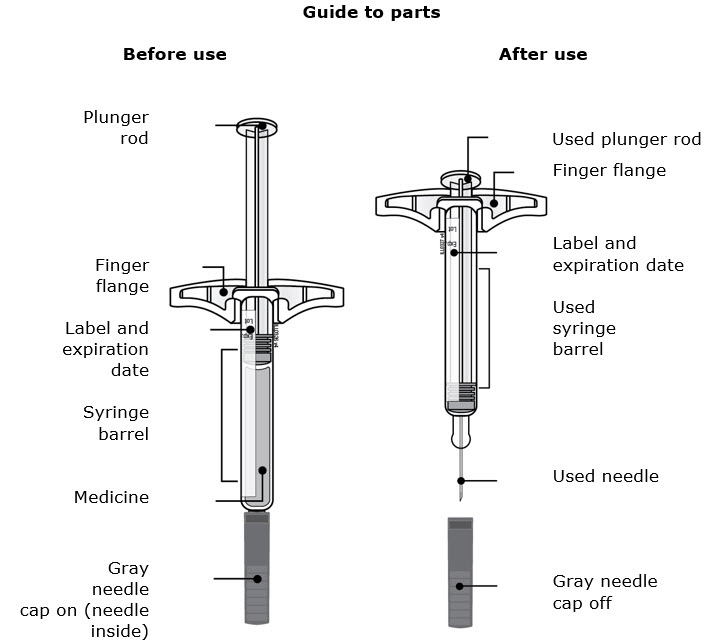
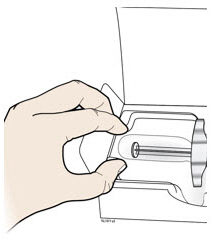
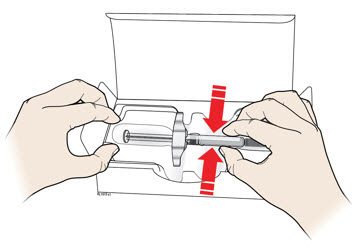
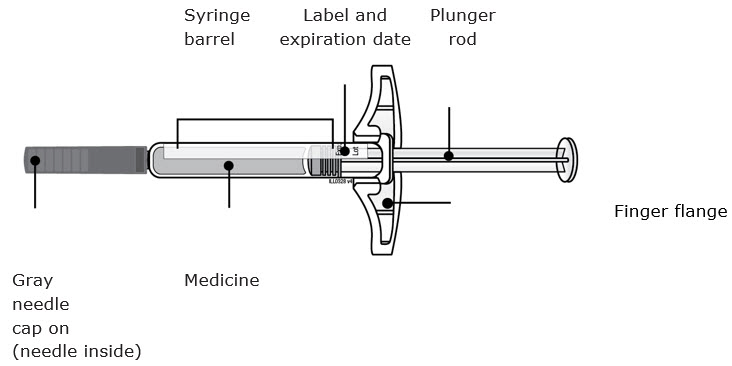
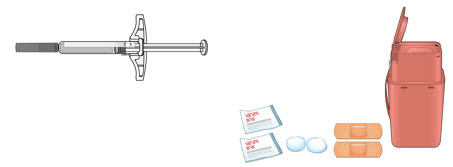
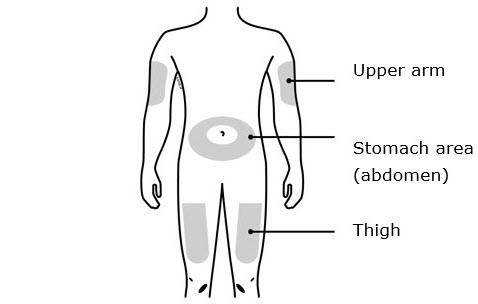
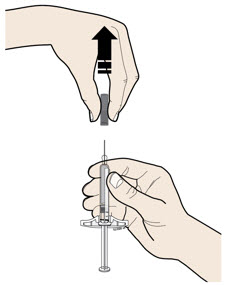
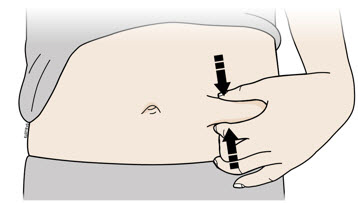
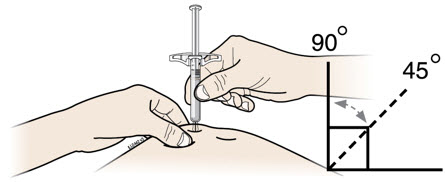
Instructions for Use AIMOVIG® (AIM-oh-vig) (erenumab-aooe) Injection, For Subcutaneous Use Single-Dose Prefilled Syringe70 mg/mL and 140 mg/mLImportant: Needle is inside the gray needle cap. Important - Before you use AIMOVIG prefilled syringe, read this important information: Storing your AIMOVIG prefilled syringe - Keep the ...
-
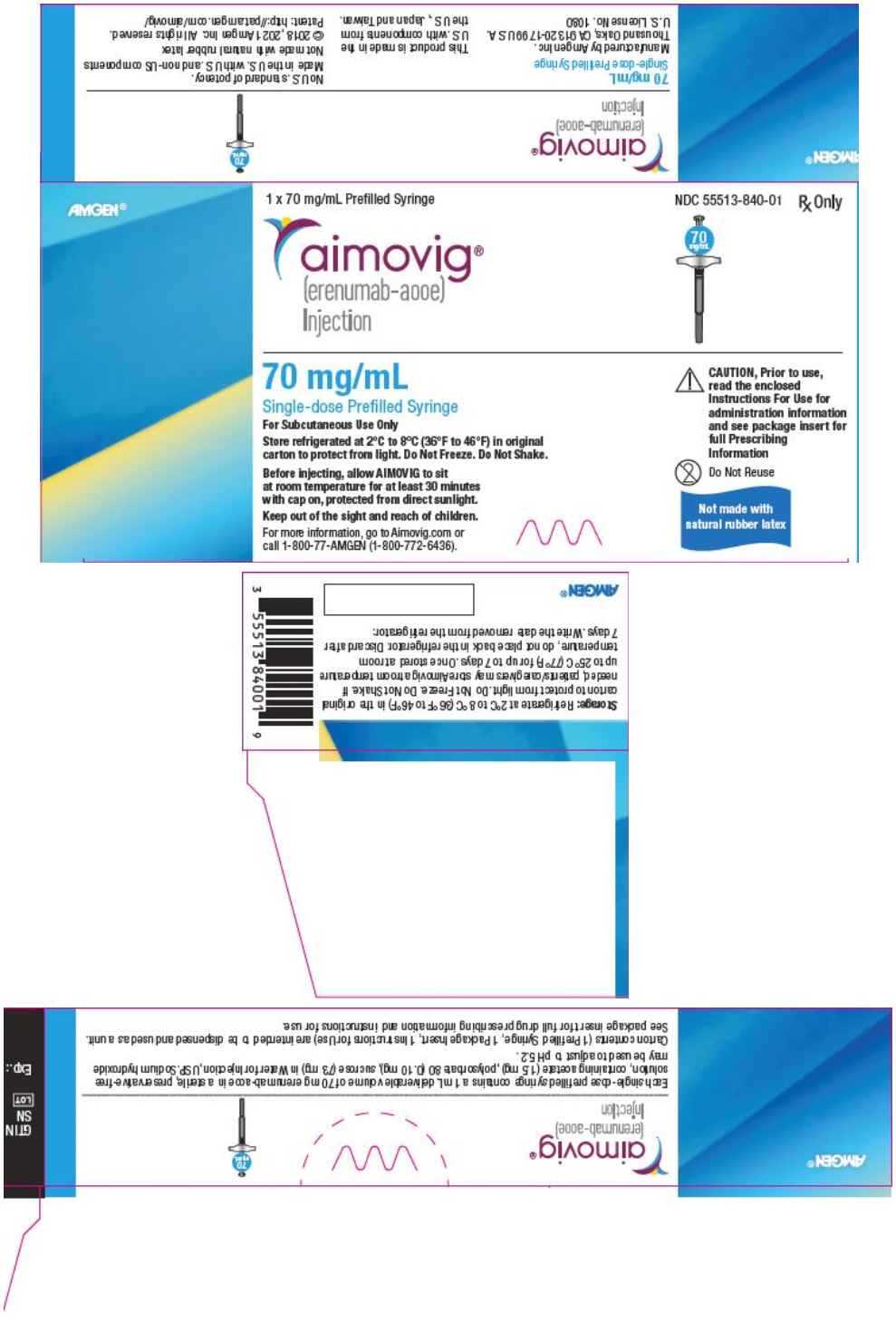
PRINCIPAL DISPLAY PANEL - 70 mg/mL Syringe CartonAMGEN® 1 x 70 mg/mL Prefilled Syringe - NDC 55513-840-01 - Rx Only - aimovig® (erenumab-aooe) Injection - 70 - mg/mL - 70 mg/mL - Single-dose Prefilled Syringe - For Subcutaneous Use Only - Store refrigerated at ...
-
PRINCIPAL DISPLAY PANEL - 70 mg/mL Autoinjector CartonAMGEN® 1 x 70 mg/mL Prefilled Autoinjector - NDC 55513-841-01 - Rx Only - aimovig® (erenumab-aooe) Injection - 70 - mg/mL - 70 mg/mL - Prefilled Autoinjector - For Subcutaneous Use Only - Store refrigerated at ...
-
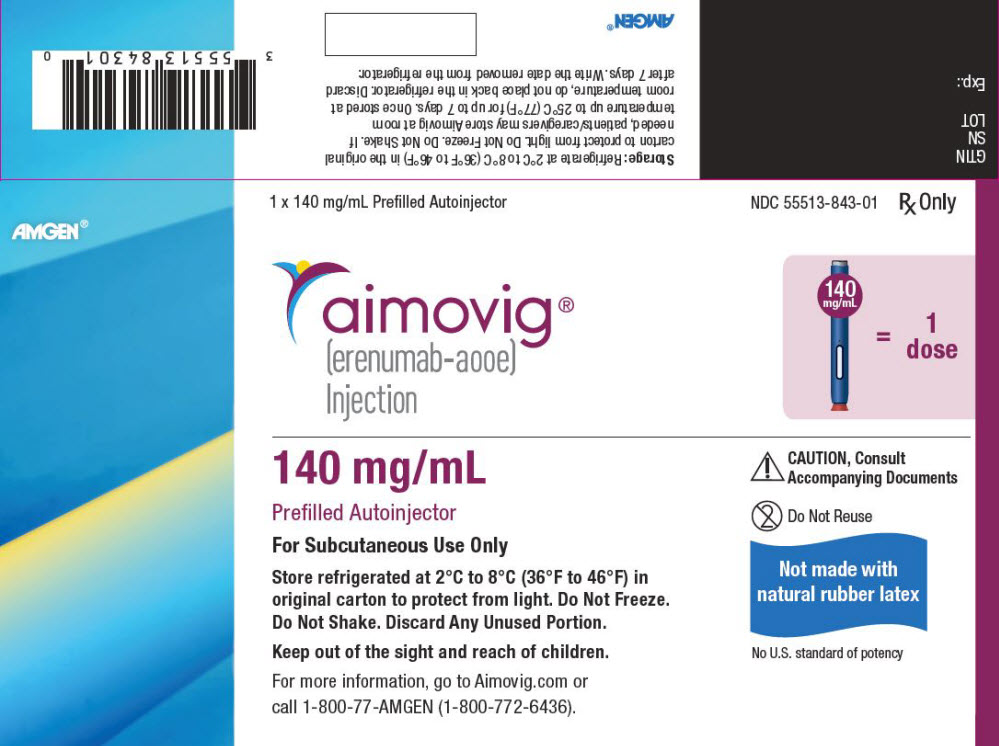
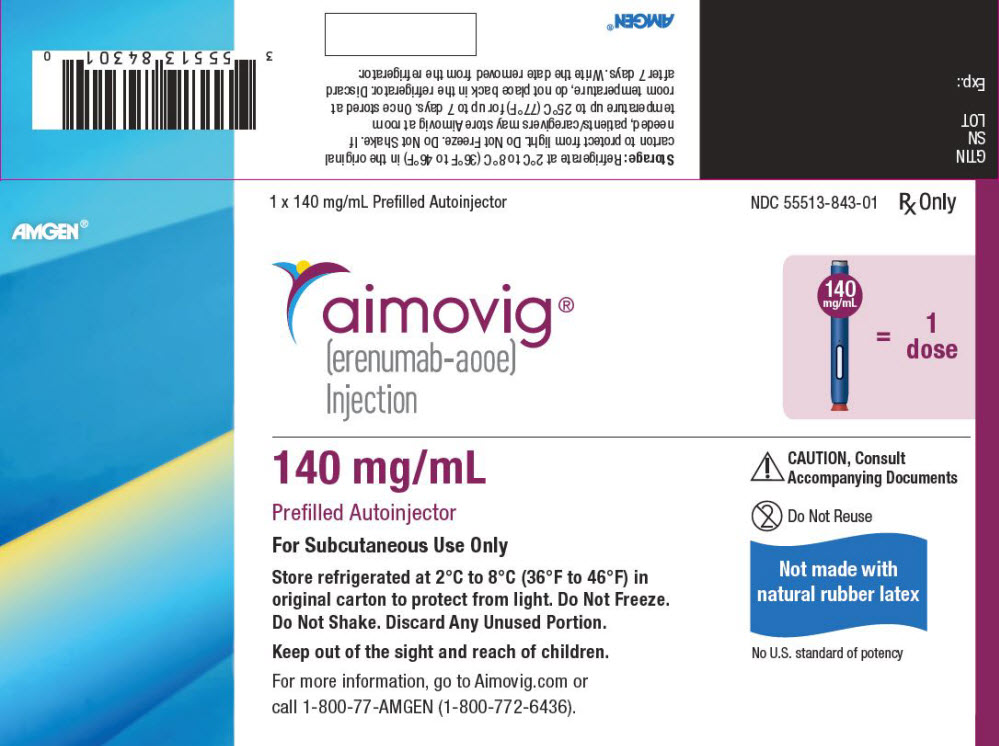
PRINCIPAL DISPLAY PANEL - 140 mg/mL Autoinjector CartonAMGEN® 1 x 140 mg/mL Prefilled Autoinjector - NDC 55513-843-01 - Rx Only - aimovig® (erenumab-aooe) Injection - 140 - mg/mL = 1 - dose - 140 mg/mL - Prefilled Autoinjector - For Subcutaneous Use Only - Store ...
-
PRINCIPAL DISPLAY PANEL - 140 mg/mL Syringe CartonAMGEN® 1 x 140 mg/mL Prefilled Syringe - NDC 55513-842-01 - Rx Only - aimovig® (erenumab-aooe) Injection - 140 - mg/mL = 1 - dose - 140 mg/mL - Single-dose Prefilled Syringe - For Subcutaneous Use Only - Store ...
-
INGREDIENTS AND APPEARANCEProduct Information










 This printed material is recyclable.
This printed material is recyclable. 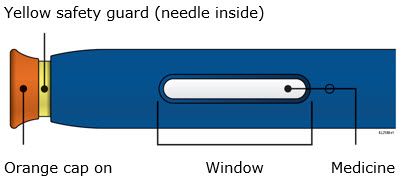




 This printed material is recyclable.
This printed material is recyclable.