Label: AFREZZA- insulin human powder, metered
AFREZZA- insulin human kit
-
NDC Code(s):
47918-874-90,
47918-878-90,
47918-880-18,
47918-891-90, view more47918-898-18, 47918-902-18, 47918-903-27
- Packager: Mannkind Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AFREZZA® safely and effectively. See full prescribing information for AFREZZA. AFREZZA® (insulin human) inhalation powder, for oral ...These highlights do not include all the information needed to use AFREZZA® safely and effectively. See full prescribing information for AFREZZA.
AFREZZA® (insulin human) inhalation powder, for oral inhalation use
Initial U.S. Approval: 2014WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
See full prescribing information for complete boxed warning.
- Acute bronchospasm has been observed in AFREZZA-treated patients with asthma and COPD. (5.1)
- AFREZZA is contraindicated in patients with chronic lung disease such as asthma or COPD. (4)
- Before initiating AFREZZA, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients. (2.5), (5.1)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Only administer via oral inhalation using the AFREZZA inhaler (2.2)
- Administer at the beginning of each meal (2.2)
- See full prescribing information for the recommended starting mealtime dosage in insulin-naïve patients and patients who are using subcutaneous mealtime insulin or pre-mixed insulin (2.3)
- Modify dosage based on the patient's metabolic needs, blood glucose monitoring results, and glycemic control goal (2.3)
- If blood glucose control is not achieved in patients requiring high AFREZZA doses, consider discontinuing AFREZZA (2.4)
DOSAGE FORMS AND STRENGTHS
Inhalation powder in single-use cartridges of: 4 units, 8 units, or 12 units (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypoglycemia or Hyperglycemia with Changes in Insulin Regimen: Carry out under close medical supervision and increase frequency of blood glucose monitoring. For patients with type 2 diabetes mellitus, oral antidiabetic treatment dosage modifications may be needed. (5.2)
- Hypoglycemia (may be life-threatening): Increase frequency of glucose monitoring in patients at higher risk for hypoglycemia and those who have reduced symptomatic awareness of hypoglycemia. (5.3)
- Decline in Pulmonary Function: Assess pulmonary function (e.g., spirometry (FEV1)) before initiating, after 6 months of therapy, and annually, even in the absence of pulmonary symptoms. In patients who have a decline of ≥ 20% in FEV1 from baseline, consider discontinuing AFREZZA. (2.5, 5.4)
- Lung Cancer: In patients with active lung cancer, a prior history of lung cancer, or in patients at risk for lung cancer, consider whether the benefits of AFREZZA use outweigh this potential risk. (5.5)
- Diabetic Ketoacidosis: In patients at risk for DKA, increase the frequency of glucose monitoring and consider changing to alternate route of insulin delivery. (5.6)
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including AFREZZA. If hypersensitivity reactions occur, discontinue AFREZZA, treat per standard of care and monitor until symptoms and signs resolve. (5.7)
- Hypokalemia (may be life-threatening): Monitor potassium levels in patients at risk of hypokalemia. (5.8)
- Fluid Retention and Heart Failure with Concomitant Use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs. (5.9)
ADVERSE REACTIONS
The most common adverse reactions associated with AFREZZA (2% or greater incidence) are hypoglycemia, cough, and throat pain or irritation (6)
To report SUSPECTED ADVERSE REACTIONS, contact MannKind at 1-877-323-8505 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2023
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Lung Function Assessment Prior to Administration
2.2 Important Administration and Discard Instructions
2.3 Recommended Starting Mealtime Dosage
2.4 Mealtime Dosage Modification
2.5 Dosage Modifications for Drug Interactions
2.6 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 Warnings and Precautions
5.1 Acute Bronchospasm in Patients with Chronic Lung Disease
5.2 Hypoglycemia or Hyperglycemia with Changes in Insulin Regimen
5.3 Hypoglycemia
5.4 Decline in Pulmonary Function
5.5 Lung Cancer
5.6 Diabetic Ketoacidosis
5.7 Hypersensitivity Reactions
5.8 Hypokalemia
5.9 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs That May Increase the Risk of Hypoglycemia
7.2 Drugs That May Decrease the Blood Glucose Lowering Effect of AFREZZA
7.3 Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of AFREZZA
7.4 Drugs That May Affect Hypoglycemia Signs and Symptoms
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
11.1 AFREZZA Cartridges
11.2 AFREZZA Inhaler
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies of AFREZZA in Adults for Diabetes Mellitus
14.2 Adults with Type 1 Diabetes
14.3 Adults with Type 2 Diabetes
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
- Acute bronchospasm has been observed in AFREZZA-treated patients with asthma and COPD [see Warnings and Precautions (5.1)].
- AFREZZA is contraindicated in patients with chronic lung disease such as asthma or COPD [see Contraindications (4)].
- Before initiating AFREZZA, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients [see Dosage and Administration (2.5), Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
AFREZZA® is a rapid acting inhaled human insulin indicated to improve glycemic control in adult patients with diabetes mellitus. Limitations of Use: AFREZZA is not recommended for the ...
AFREZZA® is a rapid acting inhaled human insulin indicated to improve glycemic control in adult patients with diabetes mellitus.
Limitations of Use:
- AFREZZA is not recommended for the treatment of diabetic ketoacidosis [see Warning and Precautions (5.6)].
- The safety and effectiveness of AFREZZA in patients who smoke have not been established. The use of AFREZZA is not recommended in patients who smoke or who have recently stopped smoking.
-
2 DOSAGE AND ADMINISTRATION
2.1 Lung Function Assessment Prior to Administration - AFREZZA is contraindicated in patients with chronic lung disease because of the risk of acute bronchospasm in these patients. Before ...
2.1 Lung Function Assessment Prior to Administration
AFREZZA is contraindicated in patients with chronic lung disease because of the risk of acute bronchospasm in these patients. Before initiating AFREZZA, perform a medical history, physical examination and spirometry (FEV1) in all patients to identify potential lung disease [see Contraindications (4) and Warnings and Precautions (5.1)].
2.2 Important Administration and Discard Instructions
Only administer AFREZZA via oral inhalation using the AFREZZA Inhaler. Administer AFREZZA at the beginning of each meal. Administer AFREZZA using a single inhalation per cartridge (if the dose is greater than the contents of a single cartridge, more than one cartridge is needed) [see Dosage and Administration (2.3)]. For additional administration instructions on how to use the AFREZZA Inhaler [see Dosage and Administration (2.6)] and see the Instructions for Use.
The AFREZZA Inhaler can be used for up to 15 days from the date of first use. After 15 days of use, the inhaler must be discarded and replaced with a new inhaler.
2.3 Recommended Starting Mealtime Dosage
For insulin naïve patients, start on 4 units of AFREZZA at the beginning of each meal.
For patients using subcutaneous, mealtime (prandial) insulin:
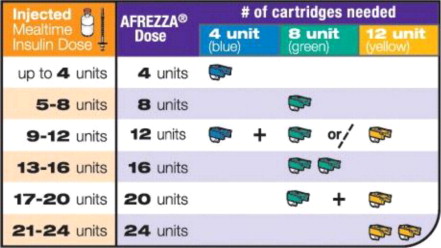
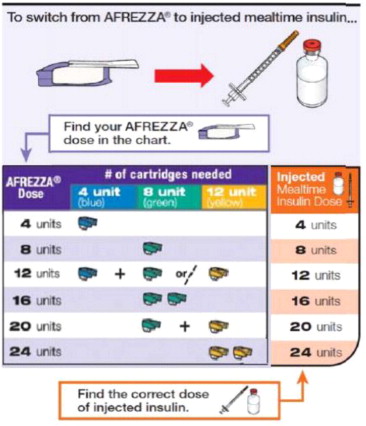
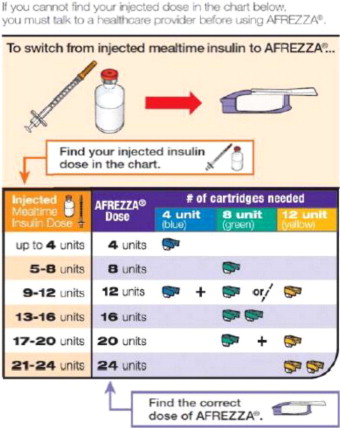
- Determine the appropriate AFREZZA dose for each meal by converting from the injected insulin dose using Figure 1.
- When switching from another insulin to AFREZZA, a different insulin dose may be needed requiring increased frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
For patients using subcutaneous, pre-mixed insulin:
- Estimate the mealtime injected dose by dividing half of the total daily injected pre-mixed insulin dose equally among the three meals of the day. When switching from another insulin to AFREZZA, a different insulin dose may be needed. When switching a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- Convert each estimated injected mealtime dose to an appropriate AFREZZA dose using Figure 1.
- Administer half of the total daily injected pre-mixed dose as an injected basal insulin dose.
For AFREZZA doses exceeding the contents of a single cartridge at mealtime, inhalations from more than one cartridge are necessary. To achieve the required total mealtime dose, use a combination of 4 unit, 8 unit, and 12 unit cartridges. Examples of cartridge combinations for doses of up to 24 units are shown in Figure 1. For doses above 24 units, use combinations of different multiple cartridges.
2.4 Mealtime Dosage Modification
- Modify the AFREZZA dosage based on the patient's metabolic needs, blood glucose monitoring results, and glycemic control goal.
- Dosage modifications may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness [see Warnings and Precautions (5.3) and Use in Specific Populations (8.6, 8.7)].
- Carefully monitor blood glucose control in patients requiring high AFREZZA doses. If blood glucose control is not achieved with increased AFREZZA doses in these patients, consider discontinuing AFREZZA.
Close2.6 Administration Instructions
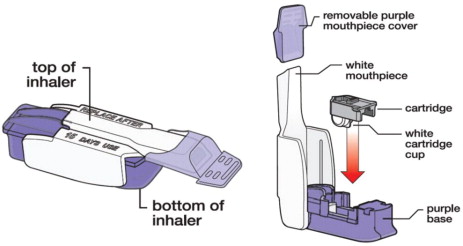
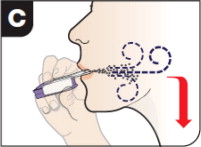
Refer patients to the Instructions for Use for detailed instructions and visuals on how to prepare, administer, and store AFREZZA; use the AFREZZA cartridges; and use the AFREZZA inhaler. Critical administration instructions are as follows:
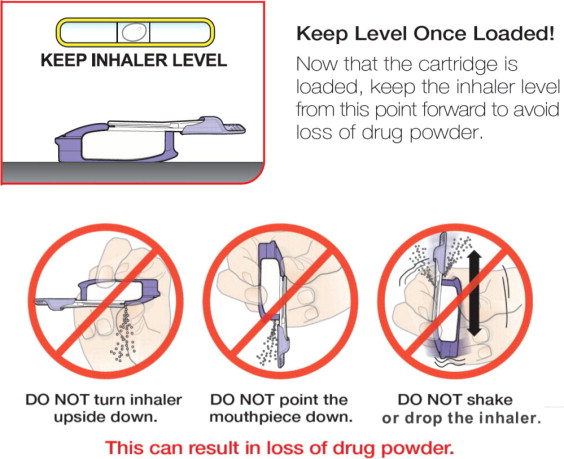
- Keep the inhaler level with the white mouthpiece on top and purple base on the bottom after a cartridge has been inserted into the inhaler. Loss of drug effect can occur if the inhaler is turned upside down, held with the mouthpiece pointing down, shaken, or dropped after the cartridge has been inserted but before the dose has been administered. If any of the above occur, replace the cartridge before use.
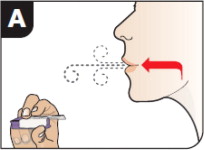
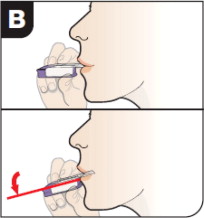
- Hold the inhaler away from the mouth and fully exhale.
- After the inhaler is placed in the mouth and the lips form a seal, tilt the inhaler down towards the chin while keeping the head level.
- With the mouth closed around the mouthpiece, inhale deeply through the inhaler.
- Hold the breath for as long as comfortable and at the same time remove the inhaler from the mouth.
- After holding the breath, exhale and continue to breathe normally.
-
3 DOSAGE FORMS AND STRENGTHS
Inhalation Powder: single-use cartridges containing 4 units, 8 units or 12 units of insulin human as white powder to be administered via oral inhalation with the AFREZZA inhaler only [see How ...
-
4 CONTRAINDICATIONS
AFREZZA is contraindicated: During episodes of hypoglycemia [see Warning and Precautions (5.3)]. Chronic lung disease, such as asthma or chronic obstructive pulmonary disease (COPD), because of ...
AFREZZA is contraindicated:
- During episodes of hypoglycemia [see Warning and Precautions (5.3)].
- Chronic lung disease, such as asthma or chronic obstructive pulmonary disease (COPD), because of the risk of acute bronchospasm [see Warnings and Precautions (5.1)]
- Hypersensitivity to regular human insulin or any of the excipients in AFREZZA [see Warnings and Precautions (5.7)]
-
5 Warnings and Precautions
5.1 Acute Bronchospasm in Patients with Chronic Lung Disease - Because of the risk of acute bronchospasm, AFREZZA is contraindicated in patients with chronic lung disease such as asthma or ...
5.1 Acute Bronchospasm in Patients with Chronic Lung Disease
Because of the risk of acute bronchospasm, AFREZZA is contraindicated in patients with chronic lung disease such as asthma or COPD [see Contraindications (4)]. Before initiating therapy with AFREZZA, evaluate all patients with a medical history, physical examination, and spirometry (FEV1) to identify potential underlying lung disease.
Acute bronchospasm has been observed in AFREZZA-treated patients with asthma and COPD. In a study of patients with asthma whose bronchodilators were temporarily withheld for assessment, bronchoconstriction and wheezing following AFREZZA dosing was reported in 29% (5 out of 17) and 0% (0 out of 13) of patients with and without a diagnosis of asthma, respectively. In this study, a mean decline in FEV1 of 400 mL was observed 15 minutes after a single AFREZZA dose in patients with asthma. In a subset study of patients with COPD (n=8), a mean decline in FEV1 of 200 mL was observed 18 minutes after a single AFREZZA dose.
5.2 Hypoglycemia or Hyperglycemia with Changes in Insulin Regimen
Glucose monitoring is essential for patients receiving insulin therapy. Changes in insulin strength, manufacturer, type, or method of administration may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. These changes should be made under close medical supervision and the frequency of blood glucose monitoring should be increased. For patients with type 2 diabetes, dosage modifications of concomitant oral antidiabetic treatment may be needed [see Drug Interactions (7.1, 7.2, and 7.3)].
5.3 Hypoglycemia
Glucose monitoring is essential for patients receiving insulin therapy. Hypoglycemia is the most common adverse reaction associated with insulins, including AFREZZA. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
The timing of hypoglycemia usually reflects the time-action profile of the administered insulin formulation. AFREZZA has a distinct time action profile [see Clinical Pharmacology (12)], which impacts the timing of hypoglycemia. Hypoglycemia can happen suddenly, and symptoms may differ across patients and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using certain medications [see Drug Interactions (7.4)], or in patients who experience recurrent hypoglycemia.
Factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [see Drug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers should be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Decline in Pulmonary Function
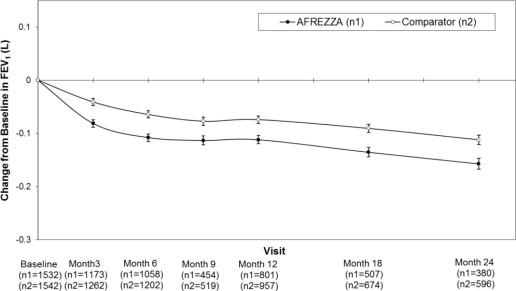
AFREZZA causes a decline in pulmonary function over time as measured by FEV1. In clinical trials excluding patients with chronic lung disease and lasting up to 2 years, AFREZZA-treated patients experienced a small [40 mL (95% CI: -80, -1)] but greater FEV1 decline than comparator-treated patients. The FEV1 decline was noted within the first 3 months, and persisted for the entire duration of therapy (up to 2 years of observation). In this population, the annual rate of FEV1 decline did not appear to worsen with increased duration of use. The effects of AFREZZA on pulmonary function for treatment duration longer than 2 years has not been established. There are insufficient data in long term studies to draw conclusions regarding reversal of the effect on FEV1 after discontinuation of AFREZZA. The observed changes in FEV1 were similar in patients with type 1 and type 2 diabetes.
Assess pulmonary function (e.g., spirometry) at baseline, after the first 6 months of therapy, and annually thereafter, even in the absence of pulmonary symptoms. In patients who have a decline of ≥ 20% in FEV1 from baseline, consider discontinuing AFREZZA. Consider more frequent monitoring of pulmonary function in patients with pulmonary symptoms such as wheezing, bronchospasm, breathing difficulties, or persistent or recurring cough. If symptoms persist, discontinue AFREZZA [see Adverse Reactions (6)].
5.5 Lung Cancer
In clinical trials, two cases of lung cancer, one in controlled trials and one in uncontrolled trials (2 cases in 2,750 patient-years of exposure), were observed in patients exposed to AFREZZA while no cases of lung cancer were observed in patients exposed to comparators (0 cases in 2,169 patient-years of exposure). In both cases, a prior history of heavy tobacco use was identified as a risk factor for lung cancer. Two additional cases of lung cancer (squamous cell and lung blastoma) occurred in non-smokers exposed to AFREZZA and were reported by investigators after clinical trial completion. These data are insufficient to determine whether AFREZZA has an effect on lung or respiratory tract tumors.
In patients with active lung cancer, a prior history of lung cancer, or in patients at risk for lung cancer, consider whether the benefits of AFREZZA use outweigh this potential risk.
5.6 Diabetic Ketoacidosis
In clinical trials enrolling patients with type 1 diabetes, diabetic ketoacidosis (DKA) was more common in AFREZZA-treated patients (0.43%; n=13) than in comparator-treated patients (0.14%; n=3). Patients with type 1 diabetes should always use AFREZZA in combination with basal insulin. In patients at risk for DKA, such as those with an acute illness or infection, increase the frequency of glucose monitoring and consider discontinuing AFREZZA and giving insulin using an alternate route of administration.
5.7 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including AFREZZA.
If hypersensitivity reactions occur, discontinue AFREZZA, treat per standard of care and monitor until symptoms and signs resolve [see Adverse Reactions (6)]. AFREZZA is contraindicated in patients who have had hypersensitivity reactions to AFREZZA or any of its excipients [see Contraindications (4)].
5.8 Hypokalemia
All insulin products, including AFREZZA, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death.
Monitor potassium levels in AFREZZA-treated patients at risk for hypokalemia (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations and patients receiving intravenously administered insulin).
Close5.9 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure.
Patients treated with insulin, including AFREZZA, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist should be considered.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling: Acute bronchospasm in patients with chronic lung disease [see Warnings and Precautions ...
The following serious adverse reactions are described below and elsewhere in the labeling:
- Acute bronchospasm in patients with chronic lung disease [see Warnings and Precautions (5.1)]
- Hypoglycemia [see Warnings and Precautions (5.3)]
- Decline in pulmonary function [see Warnings and Precautions (5.4)]
- Lung cancer [see Warnings and Precautions (5.5)]
- Diabetic ketoacidosis [see Warnings and Precautions (5.6)]
- Hypersensitivity reactions [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure of 3,017 patients to AFREZZA and include 1,026 patients with type 1 diabetes and 1,991 patients with type 2 diabetes. The mean exposure duration was 8.2 months for patients with type 1 diabetes and those with type 2 diabetes. In the overall population:
- 1,874 patients were exposed to AFREZZA for 6 months and 724 patients for greater than one year.
- 620 and 1,254 patients with type 1 and type 2 diabetes, respectively, were exposed to AFREZZA for up to 6 months.
- 238 and 486 patients with type 1 and type 2 diabetes, respectively, were exposed to AFREZZA for greater than one year (median exposure = 1.8 years).
AFREZZA was studied in placebo and active-controlled trials (n = 3 and n = 10, respectively).
The mean age of the population was 50.2 years and 20 patients were older than 75 years of age; 51% of the population were males; 83% were White, 5% were Black or African American, and 2% were Asian; 10% were Hispanic. At baseline, the type 1 diabetes population had diabetes for an average of 16.6 years and had a mean HbA1c of 8.3%, and the type 2 diabetes population had diabetes for an average of 10.7 years and had a mean HbA1c of 8.8%. At baseline, 33% of the population reported peripheral neuropathy, 32% reported retinopathy and 20% had a history of cardiovascular disease.
Table 1 shows the frequency of common adverse reactions, excluding hypoglycemia, associated with the use of AFREZZA in the pool of controlled trials in type 2 diabetes patients. These adverse reactions were not present at baseline, occurred more commonly on AFREZZA than on placebo and/or comparator and occurred in at least 2% of patients treated with AFREZZA.
Table 1. Common Adverse Reactions That Occurred in ≥ 2% in Patients with Type 2 Diabetes Mellitus (excluding Hypoglycemia) Treated with AFREZZA *Carrier particle without insulin was used as placebo [see Description (11.1)].
AFREZZA
(n = 1,991)Placebo*
(n = 290)Non-placebo comparators (n=1,363) Cough 25.6%
4.4%
3.1%
2.7%
2.2%
2.0%
2.0%19.7%
3.8%
2.8%
1.4%
1.0%
0.7%
0.3%5.4%
0.9%
1.8%
2.2%
0.9%
0.6%
1.0%Throat pain or irritation Headache Diarrhea Productive cough Fatigue Nausea Table 2 shows the frequency of common adverse reactions, excluding hypoglycemia, associated with the use of AFREZZA in the pool of active-controlled trials in type 1 diabetes patients. These adverse reactions were not present at baseline, occurred more commonly on AFREZZA than on comparator, and occurred in at least 2% of patients treated with AFREZZA.
Table 2. Common Adverse Reactions That Occurred in ≥ 2% in Patients with Type 1 Diabetes Mellitus (excluding Hypoglycemia) Treated with AFREZZA AFREZZA
(n=1026)Subcutaneous Insulin
(n = 835)Cough 29.4% 4.9% Throat pain or irritation 5.5% 1.9% Headache 4.7% 2.8% Pulmonary function test decreased 2.8% 1.0% Bronchitis 2.5% 2.0% Urinary tract infection 2.3% 1.9% Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including AFREZZA [see Warnings and Precautions (5.3)]. The incidence of severe and non-severe hypoglycemia in AFREZZA-treated patients versus placebo-treated patients with type 2 diabetes is shown in Table 3. A hypoglycemic episode was recorded if a patient reported symptoms of hypoglycemia with or without a blood glucose value consistent with hypoglycemia. Severe hypoglycemia was defined as an event with symptoms consistent with hypoglycemia requiring the assistance of another person and associated with either a blood glucose value consistent with hypoglycemia or prompt recovery after treatment for hypoglycemia.
Table 3. Incidence of Severe and Non-Severe Hypoglycemia in a Placebo-Controlled Study of Patients with Type 2 Diabetes AFREZZA
(N=177)Placebo
(N=176)Severe Hypoglycemia 5.1% 1.7% Non-Severe Hypoglycemia 67% 30% Cough
Approximately 27% of patients treated with AFREZZA reported cough, compared to approximately 5% of patients treated with comparator. In clinical trials, cough was the most common reason for discontinuation of AFREZZA therapy (3% of AFREZZA-treated patients).
Pulmonary Function Decline
In clinical trials lasting up to 2 years, excluding patients with chronic lung disease, patients treated with AFREZZA had a 40 mL (95% CI: -80, -1) greater decline from baseline in forced expiratory volume in one second (FEV1) compared to patients treated with comparator anti-diabetes treatments. The decline occurred during the first 3 months of therapy and persisted over 2 years (Figure 2). A decline in FEV1 of ≥ 15% occurred in 6% of AFREZZA-treated patients compared to 3% of comparator-treated patients [see Warnings and Precautions (5.4)].
Figure 2. Mean (+/-SE) Change in FEV1 (Liters) from Baseline for Type 1 and Type 2 Diabetes Patients
Weight Gain
Weight gain has occurred with some insulin therapies, including AFREZZA. Weight gain has been attributed to the anabolic effects of insulin and the decrease in glycosuria. In a clinical trial of patients with type 2 diabetes [see Clinical Studies (14.3)], there was a mean 0.49 kg weight gain among AFREZZA-treated patients compared with a mean 1.13 kg weight loss among placebo-treated patients.
Close6.2 Postmarketing Experience
The following adverse reaction has been identified during post approval use of AFREZZA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: bronchospasm.
-
7 DRUG INTERACTIONS
7.1 Drugs That May Increase the Risk of Hypoglycemia - The risk of hypoglycemia associated with AFREZZA use may be increased with antidiabetic agents, ACE inhibitors, angiotensin II receptor ...
7.1 Drugs That May Increase the Risk of Hypoglycemia
The risk of hypoglycemia associated with AFREZZA use may be increased with antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics. Dose modification and increased frequency of glucose monitoring may be required when AFREZZA is given concomitantly with these drugs.
7.2 Drugs That May Decrease the Blood Glucose Lowering Effect of AFREZZA
The glucose lowering effect of AFREZZA may be decreased when given concomitantly with atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline) and thyroid hormones. Dose adjustment and increased frequency of glucose monitoring may be required when AFREZZA is given concomitantly with these drugs.
7.3 Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of AFREZZA
The glucose lowering effect of AFREZZA may be increased or decreased when co-administered with alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose modification and increased frequency of glucose monitoring may be required when AFREZZA is given concomitantly with these drugs.
Close7.4 Drugs That May Affect Hypoglycemia Signs and Symptoms
The signs and symptoms of hypoglycemia may be blunted when beta-blockers, clonidine, guanethidine, and reserpine are given concomitantly with AFREZZA.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Limited available data with AFREZZA use in pregnant women are insufficient to determine drug-associated risks for adverse developmental outcomes. Available ...
8.1 Pregnancy
Risk Summary
Limited available data with AFREZZA use in pregnant women are insufficient to determine drug-associated risks for adverse developmental outcomes. Available information from published studies with human insulin use during pregnancy has not reported a clear association with human insulin and adverse developmental outcomes (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations). In animal reproduction studies, there were no adverse developmental outcomes with subcutaneous administration of carrier particles (vehicle without insulin) to pregnant rats during organogenesis at doses 21 times the human daily dose of 99 mg AFREZZA, based on AUC (see Data).
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, stillbirth, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia- related morbidity.
Data
Human Data
There are limited data with AFREZZA use in pregnant women. Published data do not report a clear association with human insulin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when human insulin is used during pregnancy. However, these studies cannot definitely establish the absence of any risk because of methodological limitations including small sample size and lack of blinding.
Animal Data
In pregnant rats given subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier particles (vehicle without insulin) from gestation day 6 through 17 (organogenesis), no major malformations were observed at doses up to 100 mg/kg/day (21 times the human systemic exposure at a daily dose of 99 mg AFREZZA, based on AUC).
In pregnant rabbits given subcutaneous doses of 2, 10, and 100 mg/kg/day of carrier particles (vehicle without insulin) from gestation day 7 through 19 (organogenesis), adverse maternal effects were observed in all dose groups (at human systemic exposure following a daily dose of 99 mg AFREZZA, based on AUC).
In pregnant rats given subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier particles (vehicle without insulin) from gestation day 7 through lactation day 20 (weaning), decreased epididymis and testes weights were observed in F1 male offspring, however, no decrease in fertility was noted, and impaired learning were observed in F1 pups at ³ 30 mg/kg/day (6 times the human systemic exposure at a daily dose of 99 mg AFREZZA, based on AUC).
8.2 Lactation
Risk Summary
There are no data on the presence of AFREZZA in human milk, the effects on the breastfed infant, or the effects on milk production. One small published study reported that exogenous subcutaneous insulin was present in human milk. No adverse effects in infants were noted. The carrier particles are present in rat milk (see Data). Potential adverse reactions that are related to inhalational administration of AFREZZA are unlikely to be associated with potential exposure of AFREZZA through breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for AFREZZA and any potential adverse effects on the breastfed infant from AFREZZA or from the underlying maternal condition.
Data
Subcutaneous administration of the carrier particle in lactating rats resulted in excretion of the carrier particle in rat milk at levels that were approximately 10% of the maternal exposure. Given the results of the rat study, it is highly likely that the insulin and carrier in AFREZZA are excreted in human milk.
8.4 Pediatric Use
The safety and effectiveness of AFREZZA to improve glycemic control in pediatric patients with diabetes mellitus has not been established. AFREZZA has not been studied in pediatric patients.
8.5 Geriatric Use
In the AFREZZA clinical studies, 671 (12%) patients were 65 years of age or older, of which 42 (0.8%) were 75 years of age or older. In these studies, 381 (13%) of AFREZZA-treated patients were 65 years of age or older, of which 20 (0.7%) were 75 years of age or older. No overall differences in effectiveness of AFREZZA have been observed between patients 65 years of age and older and younger adult patients [see Clinical Studies (14)]. Clinical studies of AFREZZA did not include sufficient numbers of patients 65 years of age and older to determine whether there were differences in safety between these patients and younger adult patients.
Pharmacokinetic and pharmacodynamic studies to assess the effect of age on pharmacokinetics or pharmacodynamics on insulin human, respectively, have not been conducted.
8.6 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of AFREZZA has not been studied. Frequent glucose monitoring and a lower dosage may be necessary in AFREZZA-treated patients with hepatic impairment [see Warnings and Precautions (5.3)].
Close8.7 Renal Impairment
The effect of renal impairment on the pharmacokinetics of AFREZZA has not been studied. Some studies with human insulin have shown increased circulating levels of insulin in patients with renal failure. Frequent glucose monitoring and a lower dosage may be necessary in AFREZZA-treated patients with renal impairment [see Warnings and Precautions (5.3)].
-
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.8)]. Mild episodes of hypoglycemia due to insulin overdose can usually be treated with ...
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.8)].
Mild episodes of hypoglycemia due to insulin overdose can usually be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise, may be needed.
Severe episodes of hypoglycemia (due to insulin overdose) with coma, seizure, or neurologic impairment may be treated with intramuscular or subcutaneous glucagon or concentrated intravenous glucose. After apparent clinical recovery from hypoglycemia, continued observation and additional carbohydrate intake may be necessary to avoid recurrence of hypoglycemia. Hypokalemia should be corrected appropriately.
Close -
11 DESCRIPTION
11.1 AFREZZA Cartridges - Human insulin is a rapid acting human insulin produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12) ...
11.1 AFREZZA Cartridges
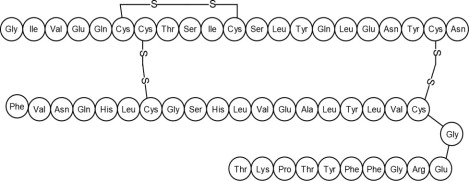
Human insulin is a rapid acting human insulin produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12). Chemically, human insulin has the empirical formula C257H383N65O77S6 and a molecular weight of 5808. Human insulin has the following primary amino acid sequence:
AFREZZA (human insulin) inhalation powder is available in single-use plastic cartridges filled with a white powder containing insulin (human), which is administered via oral inhalation using the AFREZZA Inhaler only.
Insulin is adsorbed onto carrier particles consisting of fumaryl diketopiperazine (FDKP) and polysorbate 80.
AFREZZA Inhalation Powder is a dry powder supplied as 4 unit, 8 unit or 12 unit cartridges.
Close11.2 AFREZZA Inhaler
The AFREZZA Inhaler is breath-powered by the patient. When the patient inhales through the device, the powder is aerosolized and delivered to the lung. The amount of AFREZZA delivered to the lung will depend on individual patient factors.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Insulin lowers blood glucose levels in adult patients with diabetes mellitus by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting ...
12.1 Mechanism of Action
Insulin lowers blood glucose levels in adult patients with diabetes mellitus by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis in adipocytes, inhibits proteolysis, and enhances protein synthesis.
12.2 Pharmacodynamics
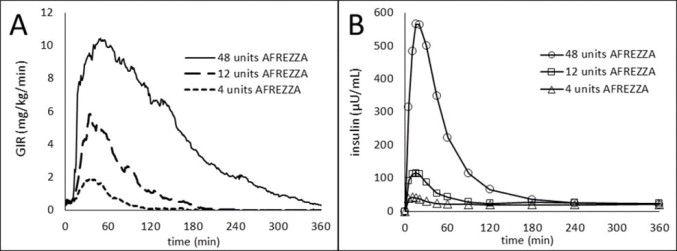
The time course of insulin action (i.e., glucose lowering) may vary considerably in different patients or within the same patient. The average pharmacodynamic profile [i.e., glucose lowering effect measured by glucose infusion rate (GIR) over time in a euglycemic clamp study] after administration of a single AFREZZA dose of 4, 12, and 48 units in 30 patients with type 1 diabetes is shown in Figure 3(A), and key characteristics regarding the timing of the effects are described in Table 4:
Table 4. Timing of Insulin Effect (i.e., mean pharmacodynamics effect) After Administration of a Single AFREZZA Dose in Patients (N=30) with Type 1 Diabetes Mellitus Parameter for Insulin Effect AFREZZA 4 units AFREZZA 12 units AFREZZA 48 units Time to first measurable effect ~12 minutes ~12 minutes ~12 minutes Time to peak effect ~35 minutes ~45 minutes ~55 minutes Time for effect to return to baseline ~90 minutes ~180 minutes ~270 minutes Figure 3. Results After Administration of AFREZZA 4, 12, and 48 Units in Patients with T1DM (N=30) A) Mean Insulin Effect (Baseline-Corrected Glucose Infusion Rate); and B) Pharmacokinetic (Baseline-Corrected Serum Insulin Concentration Profiles)
On average, the pharmacodynamics effect of AFREZZA, measured as area under the glucose infusion rate – time curve (AUC GIR) increased linearly with doses up to 48 units (106, 387, and 1581 mg/kg for 4, 12, and 48 units doses, respectively).
Intrapatient variability in AUC GIR and GIRmax was approximately 28% (95% CI 21-42%) and 27% (95% CI 20-40%), respectively.
12.3 Pharmacokinetics
The area under the plasma concentration versus time curve (AUC) of insulin increased dose proportionally up to 48 units. Intrapatient variability of AUC and peak concentration (Cmax) of insulin was approximately 16% (95% CI 12-23%) and 21% (95% CI 16-30%), respectively.
Absorption
The pharmacokinetic profiles for orally inhaled AFREZZA 4, 12, and 48 units from a study in 30 patients with type 1 diabetes are shown in Figure 5(B). The time to maximum serum insulin concentration (tmax) ranged from 10-20 minutes after oral inhalation of 4 to 48 units of AFREZZA.
Elimination
The apparent terminal half-life ranged from 120 to 206 minutes. Serum insulin concentrations declined to baseline by approximately 60 to 240 minutes.
Carrier Particles
Clinical pharmacology studies showed that carrier particles [see Description (11.1)] are not metabolized and are eliminated unchanged in the urine following the lung absorption. Following oral inhalation of AFREZZA, a mean of 39% of the inhaled dose of carrier particles was distributed to the lungs and a mean of 7% of the dose was swallowed. The swallowed fraction was not absorbed from the GI tract and was eliminated unchanged in the feces.
Drug Interaction Studies
Bronchodilators and Inhaled Steroids
Albuterol increased the AUC insulin after AFREZZA administration by 25% in patients with asthma [see Drug Interactions (7.2)]. AFREZZA is contraindicated in patients with asthma.
In a study in healthy volunteers no significant change in insulin exposure was observed when fluticasone was administered following AFREZZA administration.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of insulin human or of other insulin human products.
Increases in anti-insulin antibody concentrations were observed in patients treated with AFREZZA. Increases in anti-insulin antibodies were observed more frequently in patients treated with AFREZZA than in patients treated with subcutaneously injected mealtime insulin. There was no clinically significant effect of anti-drug antibodies on safety or effectiveness (as measured by HbA1c and fasting plasma glucose) of AFREZZA over the treatment duration of the studies which spanned 3 to 24 months.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 104 week carcinogenicity study, rats were given doses up to 46 mg/kg/day of the carrier and up to 1.23 mg/kg/day of insulin, by ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104 week carcinogenicity study, rats were given doses up to 46 mg/kg/day of the carrier and up to 1.23 mg/kg/day of insulin, by nose-only inhalation. No increased incidence of tumors was observed at systemic exposures equivalent to the insulin at a daily AFREZZA dose of 99 mg, based on a comparison of relative body surface areas across species.
No increased incidence of tumors was observed in a 26 week carcinogenicity study in transgenic mice (Tg-ras-H2) given doses up to 75 mg/kg/day of carrier and up to 5 mg/kg/day of AFREZZA.
AFREZZA was not genotoxic in Ames bacterial mutagenicity assay and in the chromosome aberration assay, using human peripheral lymphocytes with or without metabolic activation. The carrier alone was not genotoxic in the in vivo mouse micronucleus assay.
In fertility study in male and female rats at subcutaneous doses of 10, 30, and 100 mg/kg/day of carrier (vehicle without insulin), there were no adverse effects on male fertility at doses up to 100 mg/kg/day. In female rats dosed 2 weeks prior to mating until gestation day 7, there was increased pre- and post-implantation loss at 100 mg/kg/day but not at 30 mg/kg/day (21 times and 6 times, respectively the human systemic exposure at a daily dose of 99 mg AFREZZA, based on AUC).
-
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies of AFREZZA in Adults for Diabetes Mellitus - AFREZZA has been studied in adults with type 1 diabetes in combination with basal insulin. The efficacy of ...
14.1 Overview of Clinical Studies of AFREZZA in Adults for Diabetes Mellitus
AFREZZA has been studied in adults with type 1 diabetes in combination with basal insulin. The efficacy of AFREZZA, in combination with basal insulin, in type 1 diabetes patients was compared to insulin aspart in combination with basal insulin.
AFREZZA has been studied in adults with type 2 diabetes in combination with oral antidiabetic drugs. The efficacy of AFREZZA in type 2 diabetes patients was compared to placebo inhalation.
14.2 Adults with Type 1 Diabetes
Patients with inadequately controlled type 1 diabetes participated in a 24-week, open-label, active-controlled study to evaluate the glucose lowering effect of mealtime AFREZZA used in combination with a basal insulin. Following a 4-week basal insulin optimization period, 344 patients were randomized to AFREZZA by oral inhalation (n=174) or insulin aspart given subcutaneously (n=170) at each meal of the day. All patients received basal insulin. Mealtime insulin doses were titrated to glycemic goals for the first 12 weeks and kept stable for the last 12 weeks of the study.
Results
At Week 24, treatment with mealtime AFREZZA and basal insulin provided a mean reduction in HbA1c that met the pre-specified non-inferiority margin of 0.4%. AFREZZA and basal insulin provided less HbA1c reduction than insulin aspart and basal insulin, and the difference was statistically significant. More patients in the insulin aspart and basal insulin group achieved the HbA1c target of ≤7% (Table 5).
Table 5. Results at Week 24 in an Active-Controlled Study of Mealtime AFREZZA plus Basal Insulin in Adults with Type 1 Diabetes a Adjusted mean was obtained using a Mixed Model Repeated Measures (MMRM) approach with HbA1c or FPG as the dependent variable and treatment, visit, region, basal insulin stratum, and treatment by visit interaction as fixed factors, and corresponding baseline as a covariate. An autoregression (1) [AR(1)] covariance structure was used.
b Data at 24 weeks were available from 131 (75%) and 150 (88%) patients randomized to the AFREZZA and insulin aspart groups, respectively.
c The percentage was calculated based on the number of patients randomized to the trial.
Efficacy Parameter AFREZZA + Basal Insulin (N=174) Insulin Aspart + Basal Insulin
(N=170)HbA1c (%) Baseline (adjusted meana) 7.94 7.92 Change from baseline (adjusted meana,b) -0.21 -0.40 Difference from insulin aspart (adjusted meana,b)
(95% CI)0.19
(0.02, 0.36)Percentage of patients achieving HbA1c ≤ 7%c 14% 27% Fasting Plasma Glucose (mg/dL) Baseline (adjusted meana) 153.9 151.6 Change from baseline (adjusted meana, b) -25.3 10.2 Difference from insulin aspart (adjusted meana, b)
(95% CI)-35.4
(-56.3, -14.6)Close14.3 Adults with Type 2 Diabetes
A total of 479 adult patients with type 2 diabetes inadequately controlled on optimal/maximally tolerated doses of metformin only, or 2 or more oral antidiabetic (OAD) agents participated in a 24-week, double-blind, placebo-controlled study. Following a 6-week run-in period, 353 patients were randomized to AFREZZA by oral inhalation (n=177) or an inhaled placebo powder without insulin (n=176). Insulin doses were titrated for the first 12 weeks and kept stable for the last 12 weeks of the study. OADs doses were kept stable in the study.
Results
At Week 24, treatment with AFREZZA plus OADs provided a mean reduction in HbA1c that was statistically significantly greater compared to the HbA1c reduction observed in the placebo plus OADs group (Table 6).
Table 6. Results at Week 24 in a Placebo-Controlled Study of AFREZZA in Adults with Type 2 Diabetes Inadequately Controlled on Oral Antidiabetic Agents a Adjusted mean was obtained using a Mixed Model Repeated Measures (MMRM) approach with HbA1c or FPG as the dependent variable and treatment, visit, region, and treatment by visit interaction as fixed factors, and corresponding baseline as a covariate. An autoregression (1) [AR(1)] covariance structure was used.
b Data at 24 weeks without rescue therapy were available from 139 (79%) and 129 (73%) patients randomized to the AFREZZA and placebo groups, respectively.
c The percentage was calculated based on the number of patients randomized to the trial.
Efficacy Parameter AFREZZA + Oral Anti-Diabetic Agents
(N=177)Placebo + Oral Anti-Diabetic Agents
(N=176)HbA1c (%) Baseline (adjusted meana) 8.25 8.27 Change from baseline (adjusted meana,b) -0.82 -0.42 Difference from placebo (adjusted meana,b)
(95% CI)-0.40
(-0.57, -0.23)Percentage (%) of patients achieving HbA1C ≤7%c 32% 15% Fasting Plasma Glucose (mg/dL) Baseline (adjusted meana) 175.9 175.2 Change from baseline (adjusted meana,b) -11.2 -3.8 Difference from placebo (adjusted meana,b)
(95% CI)-7.4
(-18.0, 3.2) -
16 HOW SUPPLIED/STORAGE AND HANDLING
AFREZZA (insulin human) Inhalation Powder is available as 4 unit, 8 unit and 12 unit single-use cartridges. Three cartridges are contained in a single cavity of a blister strip. Each card contains ...
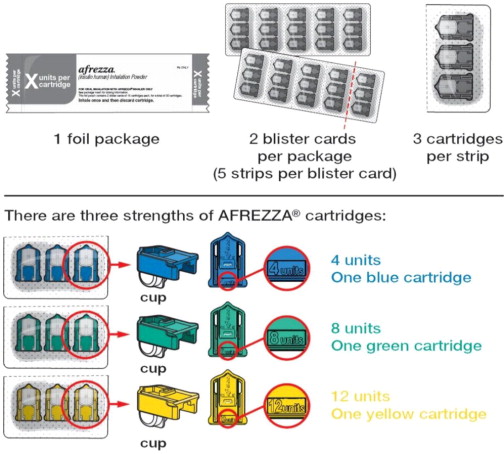
AFREZZA (insulin human) Inhalation Powder is available as 4 unit, 8 unit and 12 unit single-use cartridges. Three cartridges are contained in a single cavity of a blister strip. Each card contains 5 blister strips (each containing three cartridges) separated by perforations for a total of 15 cartridges. Two cards of the same cartridge strength are packaged in a foil laminate overwrap (30 cartridges per foil package).
The cartridges are color-coded, blue for 4 units, green for 8 units and yellow for 12 units. Each cartridge is marked with “afrezza” and “4 units”, “8 units” or “12 units”.
The AFREZZA Inhaler is individually packaged in a clear overwrap. The inhaler is fully assembled with a removable mouthpiece cover. The AFREZZA Inhaler can be used for up to 15 days from the date of first use. After 15 days of use, the inhaler must be discarded and replaced with a new inhaler.
AFREZZA (insulin human) Inhalation Powder is available in the following configurations:
NDC Cartridge Strength Quantity of Cartridges per Strength Total Quantity of Cartridges per Kit Total Units in Kit Number of Inhalers 47918-874-90 4 units 90 90 360 Units 2 47918-878-90 8 units 90 90 720 Units 2 47918-891-90 12 units 90 90 1080 Units 2 47918-898-18 8 units, 12 units 90 180 1800 Units 2 47918-880-18
(Titration Pack)4 units, 8 units 90 180 1080 Units 2 47918-902-18
(Titration Pack)4 units, 8 units, 12 units 60 180 1440 Units 2 CloseStorage:
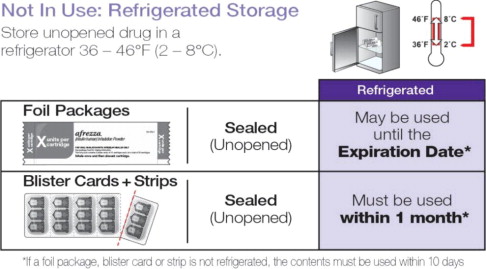
Not in Use: Refrigerated Storage 2-8ºC (36-46ºF)
* If a foil package, blister card or strip is not refrigerated, the contents must be used within 10 days.
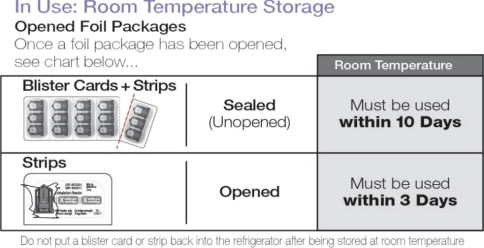
Sealed (Unopened) Foil Package May be stored until the Expiration Date* Sealed (Unopened) Blister Cards + Strips Must be used within 1 month* In Use: Room Temperature Storage 25ºC (77ºF), excursions permitted 15-30ºC (59-86ºF)
Sealed (Unopened) Blister Cards + Strips Must be used within 10 days Opened Strips Must be used within 3 days Do not put a blister card or strip back into the refrigerator after being stored at room temperature.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Instruct patients to use AFREZZA only with the AFREZZA inhaler. Common Adverse ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instruct patients to use AFREZZA only with the AFREZZA inhaler.
Common Adverse Reactions
Inform patients that the most common adverse reactions associated with the use of AFREZZA are hypoglycemia, cough, and throat pain or irritation.
Pregnancy
Advise women with diabetes to inform their physician if they are pregnant or are planning to become pregnant while using AFREZZA.
Acute Bronchospasm in Patients with Chronic Lung Disease
Advise patients that if they experience any respiratory difficulty after inhalation of AFREZZA, they should report it to their healthcare provider immediately for assessment.
Hypoglycemia
Instruct patients on self-management procedures including glucose monitoring, proper inhalation technique, and management of hypoglycemia and hyperglycemia especially at initiation of AFREZZA therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, or skipped meals.
Instruct patients on the management of hypoglycemia. Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery [see Warnings and Precautions (5.3)].
Decline in Pulmonary Function
Inform patients that AFREZZA can cause a decline in lung function [see Warnings and Precautions (5.4)].
Lung Cancer
Inform patients to promptly report any signs or symptoms potentially related to lung cancer [see Warnings and Precautions (5.5)].
Diabetic Ketoacidosis
Instruct patients to carefully monitor their blood glucose during illness, infection, and other risk situations for diabetic ketoacidosis and to contact their healthcare provider if their blood glucose control worsens [see Warnings and Precautions (5.6)].
CloseHypersensitivity Reactions
Advise patients that hypersensitivity reactions can occur with insulin therapy including AFREZZA. Inform patients on the symptoms of hypersensitivity reactions [see Warnings and Precautions (5.7)].
Manufactured by: MannKind Corporation, Danbury, CT 06810
US License No. #2190
© 2016 – 2023 MannKind CorporationAFREZZA is a registered trademark of MannKind Corporation
Patent: www.mannkindcorp.com/patent-notices -
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration - Revised 02/2023 - MEDICATION GUIDE - AFREZZA® (uh-FREZZ-uh) (insulin human) inhalation powder, for ...Close
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised 02/2023
MEDICATION GUIDE
AFREZZA® (uh-FREZZ-uh)
(insulin human)
inhalation powder, for oral inhalation useWhat is the most important information I should know about AFREZZA?
AFREZZA can cause serious side effects, including:
- Sudden lung problems (bronchospasms). Do not use AFREZZA if you have long-term (chronic) lung problems such as asthma or chronic obstructive pulmonary disease (COPD). Before starting AFREZZA, your healthcare provider will give you a breathing test to check how your lungs are working.
What is AFREZZA?
- AFREZZA is a man-made insulin that is breathed-in through your lungs (inhaled) and is used to control high blood sugar in adults with diabetes mellitus.
- AFREZZA is not for use to treat diabetic ketoacidosis. AFREZZA must be used with basal insulin in people who have type 1 diabetes mellitus.
- It is not known if AFREZZA is safe and effective for use in people who smoke. AFREZZA is not for use in people who smoke or have recently stopped smoking (less than 6 months).
- It is not known if AFREZZA is safe and effective in children under 18 years of age.
Who should not use AFREZZA?
Do not use AFREZZA if you:
- Have chronic lung problems such as asthma or COPD.
- Are allergic to regular human insulin or any of the ingredients in AFREZZA. See the end of this Medication Guide for a complete list of ingredients in AFREZZA.
- Are having an episode of low blood sugar (hypoglycemia).
What should I tell my healthcare provider before using AFREZZA?
Before using AFREZZA, tell your healthcare provider about all your medical conditions, including if you:
- Have lung problems such as asthma or COPD
- Have or have had lung cancer
- Are using any inhaled medications
- Smoke or have recently stopped smoking
- Have kidney or liver problems
- Are pregnant, planning to become pregnant, or are breastfeeding. AFREZZA may harm your unborn or breastfeeding baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins or herbal supplements.
Before you start using AFREZZA, talk to your healthcare provider about low blood sugar and how to manage it.How should I use AFREZZA?
- Read the detailed Instructions for Use that comes with your AFREZZA.
- Take AFREZZA exactly as your healthcare provider tells you to. Your healthcare provider should tell you how much AFREZZA to use and when to use it.
- Know the strength of AFREZZA you use. Do not change the amount of AFREZZA you use unless your healthcare provider tells you to.
- Take AFREZZA at the beginning of your meal.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check your blood sugar levels.
- Keep AFREZZA and all medicines out of the reach of children.
Your dose of AFREZZA may need to change because of:
- Change in level of physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
What should I avoid while using AFREZZA?
While using AFREZZA do not:
- Drive or operate heavy machinery, until you know how AFREZZA affects you
- Drink alcohol or use over-the-counter medicines that contain alcohol
- Smoke
What are the possible side effects of AFREZZA?
- AFREZZA may cause serious side effects that can lead to death, including:
See “What is the most important information I should know about AFREZZA?”
-
Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- Dizziness or light-headedness, sweating, confusion, headache, blurred vision, slurred speech, shakiness, fast heartbeat, anxiety, irritability or mood change, hunger.
- Decreased lung function. Your healthcare provider should check how your lungs are working before you start using AFREZZA, 6 months after you start using it and yearly after that.
- Lung cancer. In studies of AFREZZA in people with diabetes, lung cancer occurred in a few more people who were taking AFREZZA than in people who were taking other diabetes medications. There were too few cases to know if lung cancer was related to AFREZZA. If you have lung cancer, you and your healthcare provider should decide if you should use AFREZZA.
- Diabetic ketoacidosis. Talk to your healthcare provider if you have an illness. Your AFREZZA dose or how often you check your blood sugar may need to be changed.
-
Severe allergic reaction (whole body reaction). Get medical help right away if you have any of these signs or symptoms of a severe allergic reaction:
- A rash over your whole body, trouble breathing, a fast heartbeat, or sweating.
- Low potassium in your blood (hypokalemia).
-
Heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with AFREZZA may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with AFREZZA. Your healthcare provider should monitor you closely while you are taking TZDs with AFREZZA. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
- Shortness of breath, swelling of your ankles or feet, sudden weight gain.
Treatment with TZDs and AFREZZA may need to be changed or stopped by your healthcare provider if you have new or worse heart failure.
Get emergency medical help if you have:
- Trouble breathing, shortness of breath, fast heartbeat, swelling of your face, tongue, or throat, sweating, extreme drowsiness, dizziness, confusion.
The most common side effects of AFREZZA include:
- Low blood sugar (hypoglycemia), cough, sore throat.
These are not all the possible side effects of AFREZZA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 (1-800-332-1088). General information about the safe and effective use of AFREZZA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use AFREZZA for a condition for which it was not prescribed. Do not give AFREZZA to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about AFREZZA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about AFREZZA that is written for health professionals.What are the ingredients in AFREZZA?
Active ingredient: human insulin
Inactive ingredients: fumaryl diketopiperazine, polysorbate 80AFREZZA is a registered trademark of MannKind Corporation
Patent: www.mannkindcorp.com/patent-notices
Manufactured by: MannKind Corporation, Danbury, CT 06810
US License No. #2190
© 2016 – 2023 MannKind Corporation
For more information, go to www.AFREZZA.com or call MannKind Corporation at 1-877-323-8505. -
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - AFREZZA® (uh-FREZZ-uh) (insulin human) inhalation powder, for oral inhalation use - This “Instructions for Use” contains information on how to use AFREZZA® (insulin human ...
INSTRUCTIONS FOR USE
AFREZZA® (uh-FREZZ-uh)
(insulin human)
inhalation powder, for oral inhalation useThis “Instructions for Use” contains information on how to use AFREZZA® (insulin human) Inhalation Powder.
Read this Instructions for Use before you start using AFREZZA and each time you get a new AFREZZA Inhaler. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Your healthcare provider should show you how to use your AFREZZA Inhaler the right way before you use it for the first time.
Important information about AFREZZA:
-
AFREZZA comes in 3 strengths (see Figure A):
- 4 units (blue cartridge)
- 8 units (green cartridge)
- 12 units (yellow cartridge)
- If your prescribed AFREZZA dose is higher than 12 units, you will need to use more than 1 cartridge.
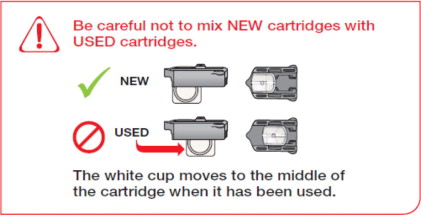
- If you need to use more than 1 cartridge for your dose, throw away the used cartridge before getting a new one. You can tell when a cartridge has been used, because the cup has moved to the center.
- Do not try to open the AFREZZA cartridges. The AFREZZA Inhaler opens the cartridge automatically during use.
- AFREZZA cartridges should only be used with the AFREZZA Inhaler. Do not try to breathe in the AFREZZA insulin powder in any other way. Do not put cartridges in your mouth and do not swallow cartridges.
- Use only 1 AFREZZA Inhaler at a time. The same inhaler should be used for the 4 unit, 8 unit or 12 unit cartridges.
- Store the inhaler in a clean, dry place with the mouthpiece cover on until your next dose.
- Throw away your AFREZZA Inhaler after 15 days and get a new one.
If you are having problems with your AFREZZA Inhaler or if it breaks and you need a new one, call 1-877-323-8505.
Know your AFREZZA Inhaler:
Know your AFREZZA cartridges:
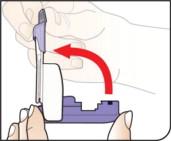
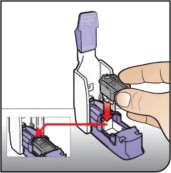
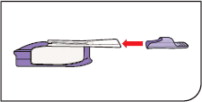
How to take your dose of AFREZZA:
Always be sure you have the right number of AFREZZA cartridges for your dose available before you start. AFREZZA cartridges must only be used with the AFREZZA Inhaler.
Step 1: Select the AFREZZA cartridges for your dose 
If your prescribed AFREZZA dose is more than 12 units you will need to use more than 1 cartridge to get your right dose.
Use the dosage chart below to determine the least number
of AFREZZA cartridges you can use for your dose. Other
cartridge combinations can be used.
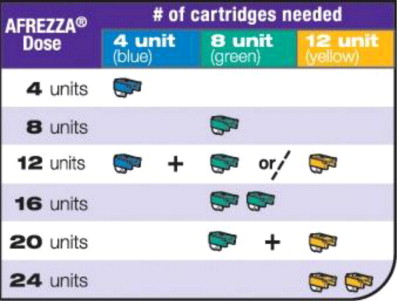 Figure B
Figure B

Select Cartridges
Important: Use the AFREZZA dose chart above (see Figure B) to help you choose the right number of AFREZZA cartridges needed for your dose.
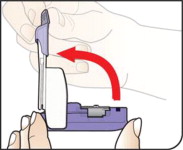
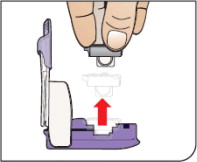
Open Packages
Remove a blister card from the foil package.
Tear along perforation to remove one strip.
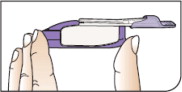
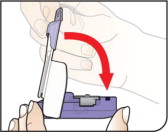
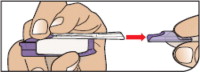
Push Cartridges to Remove
Remove a cartridge from the strip by pressing on the clear side to push the cartridge out. Remove the right number of cartridges for your dose. Pushing on the cup will not damage the cartridge.
AFREZZA cartridges left over in an opened strip must be used within 3 days.
Before Proceeding:
Check that you have the right AFREZZA cartridge(s) for your dose.
Use only 1 inhaler for multiple cartridges. Throw away your AFREZZA Inhaler after 15 days and get a new one.Multiple cartridge dosing If you need more than one (1) AFREZZA cartridge for your dose, see the AFREZZA dosage chart above (Figure B).
Repeat steps 2 through 4 for each AFREZZA cartridge you need for your prescribed AFREZZA dose.
Switching between AFREZZA and injected insulin: Contact your healthcare provider before switching insulins.
AFREZZA is a mealtime insulin.
Do not switch from AFREZZA to a long acting insulin.
Do not switch from a long acting insulin to AFREZZA.Manufactured by: MannKind Corporation
Danbury, CT 06810US License No. #2190
© 2016 – 2023 MannKind Corporation
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 02/2023
AFREZZA is a registered trademark of MannKind Corporation
Patent: www.mannkindcorp.com/patent-notices
Close -
AFREZZA comes in 3 strengths (see Figure A):
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel 90 − 4 unit cartridges and 2 inhalers - NDC 47918-874-90 - Rx ONLY - afrezza® (insulin human) Inhalation Powder - 4 units per - cartridge - 90 single-use cartridges - FOR ...
Principal Display Panel 90 − 4 unit cartridges and 2 inhalers
NDC 47918-874-90
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder4 units per
cartridge90 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
90 – 4 unit cartridges + 2 inhalers
Dispense the enclosed Medication Guide
to each patientmannkind®
Close -
PRINCIPAL DISPLAY PANELPrincipal Display Panel 90 – 8 unit cartridges and 2 inhalers - NDC 47918-878-90 - Rx ONLY - afrezza® (insulin human) Inhalation Powder - 8 units per - cartridge - 90 single-use cartridges - FOR ...
Principal Display Panel 90 – 8 unit cartridges and 2 inhalers
NDC 47918-878-90
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder8 units per
cartridge90 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
90 – 8 unit cartridges + 2 inhalers
Dispense the enclosed Medication Guide
to each patientmannkind®
Close -
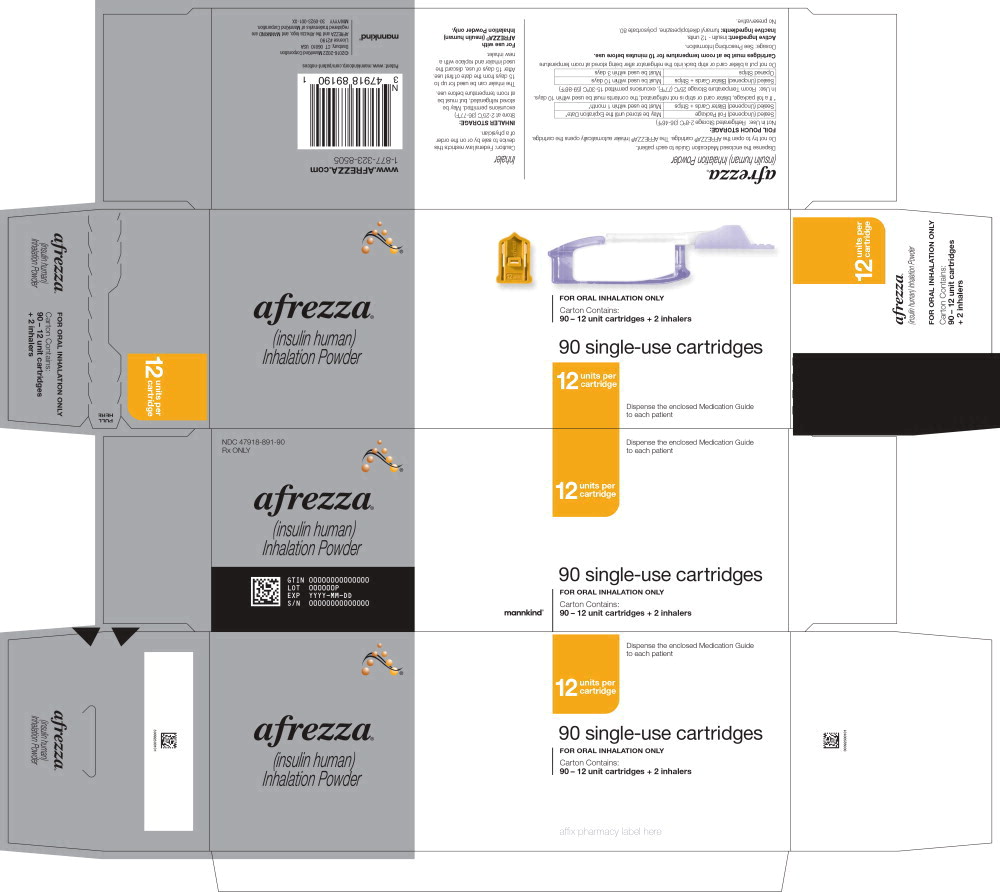
PRINCIPAL DISPLAY PANELPrincipal Display Panel 90 – 12 unit cartridges and 2 inhalers - NDC 47918-891-90 - Rx ONLY - afrezza® (insulin human) Inhalation Powder - 12 units per - cartridge - 90 single-use cartridges - FOR ...
Principal Display Panel 90 – 12 unit cartridges and 2 inhalers
NDC 47918-891-90
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder12 units per
cartridge90 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
90 – 12 unit cartridges + 2 inhalers
Dispense the enclosed Medication Guide
to each patientmannkind®
Close -
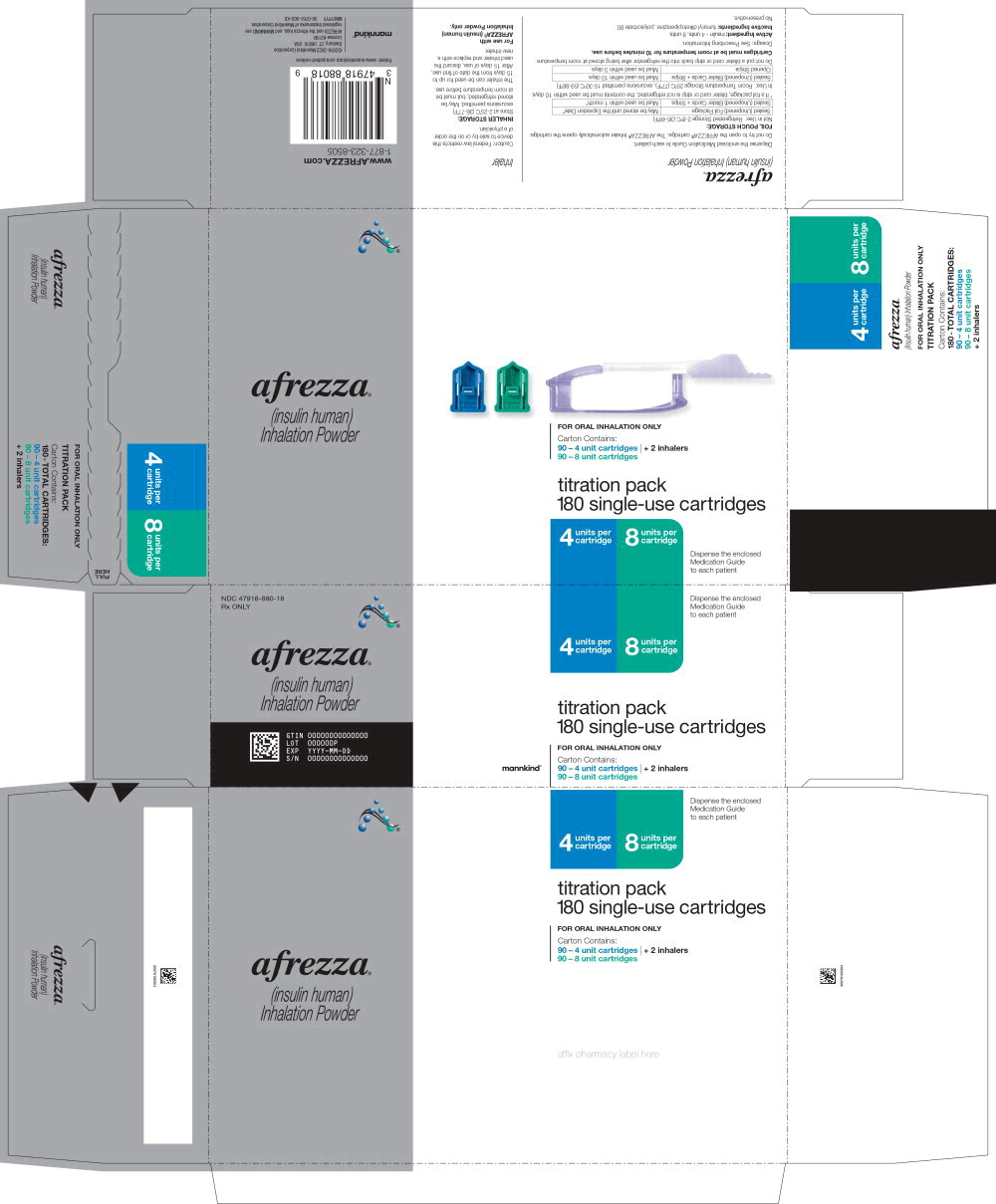
PRINCIPAL DISPLAY PANELPrincipal Display Panel 180 cartridges; 60 – 4 unit cartridges, 60 – 8 unit cartridges and 60 – 12 unit cartridges and 2 inhalers (Titration Pack) NDC 47918-902-18 - Rx ONLY - afrezza® (insulin ...
Principal Display Panel 180 cartridges; 60 – 4 unit cartridges, 60 – 8 unit cartridges and 60 – 12 unit cartridges and 2 inhalers (Titration Pack)
NDC 47918-902-18
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder4 units per
cartridge8 units per
cartridge12 units per
cartridge180 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
60 – 4 unit cartridges
60 – 8 unit cartridges
60 – 12 unit cartridges
2 - inhalers
Dispense the enclosed
Medication Guide
to each patientmannkind®
Close -
PRINCIPAL DISPLAY PANELPrincipal Display Panel 180 cartridges; 90 – 4 unit cartridges and 90 – 8 unit cartridges and 2 inhalers (Titration Pack) NDC 47918-880-18 - Rx ONLY - afrezza® (insulin human) Inhalation ...
Principal Display Panel 180 cartridges; 90 – 4 unit cartridges and 90 – 8 unit cartridges and 2 inhalers (Titration Pack)
NDC 47918-880-18
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder4 units per
cartridge8 units per
cartridgetitration pack
180 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
90 – 4 unit cartridges
90 – 8 unit cartridges
2 - inhalers
Dispense the enclosed
Medication Guide
to each patientmannkind®
Close -
PRINCIPAL DISPLAY PANELPrincipal Display Panel 180 cartridges; 90 – 8 unit cartridges and 90 – 12 unit cartridges and 2 inhalers - NDC 47918-898-18 - Rx ONLY - afrezza® (insulin human) Inhalation Powder - 8 units ...
Principal Display Panel 180 cartridges; 90 – 8 unit cartridges and 90 – 12 unit cartridges and 2 inhalers
NDC 47918-898-18
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder8 units per
cartridge12 units per
cartridge180 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
90 – 8 unit cartridges
90 – 12 unit cartridges
2 - inhalers
Dispense the enclosed
Medication Guide
to each patientmannkind®
Close -
Principal Display Panel 27 cartridges; 9 – 4 unit cartridges, 9 – 8 unit cartridges and 9 – 12 unit cartridges and 1 inhaler (Sample Kit)
NDC 47918-903-27 - Rx ONLY - afrezza® (insulin human) Inhalation Powder - 4 units per - cartridge - 8 units per - cartridge - 12 units per - cartridge - 27 single-use cartridges - FOR ORAL INHALATION ...
NDC 47918-903-27
Rx ONLY
afrezza®
(insulin human)
Inhalation Powder4 units per
cartridge8 units per
cartridge12 units per
cartridge27 single-use cartridges
FOR ORAL INHALATION ONLY
Carton Contains:
9 – 4 unit cartridges
9 – 8 unit cartridges
9 – 12 unit cartridges
1 - inhaler
Dispense the enclosed
Medication Guide
to each patientmannkind®
Close -
INGREDIENTS AND APPEARANCEProduct Information
AFREZZA insulin human powder, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-874 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 4 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-874-90 3 in 1 CARTON 01/21/2015 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 01/21/2015 AFREZZA insulin human powder, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-878 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 8 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-878-90 3 in 1 CARTON 07/01/2017 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/01/2017 AFREZZA insulin human powder, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-891 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 12 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-891-90 3 in 1 CARTON 07/01/2017 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/01/2017 AFREZZA insulin human kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-880 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-880-18 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 07/01/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 90 CARTRIDGE 90 Part 2 90 CARTRIDGE 90 Part 1 of 2 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 8 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Part 2 of 2 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 4 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/01/2016 AFREZZA insulin human kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-898 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-898-18 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 12/04/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 90 CARTRIDGE 90 Part 2 90 CARTRIDGE 90 Part 1 of 2 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 8 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Part 2 of 2 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 12 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 12/04/2018 AFREZZA insulin human kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-902 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-902-18 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 01/10/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 60 CARTRIDGE 60 Part 2 60 CARTRIDGE 60 Part 3 60 CARTRIDGE 60 Part 1 of 3 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 8 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Part 2 of 3 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 4 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Part 3 of 3 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 12 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 PACKAGE, COMBINATION 1 2 in 1 POUCH 1 15 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 01/10/2017 AFREZZA insulin human kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47918-903 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47918-903-27 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 11/09/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 9 CARTRIDGE 9 Part 2 9 CARTRIDGE 9 Part 3 9 CARTRIDGE 9 Part 1 of 3 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 8 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 9 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Part 2 of 3 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 4 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 9 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Part 3 of 3 AFREZZA insulin human powder, metered Product Information Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 12 [arb'U] Inactive Ingredients Ingredient Name Strength FUMARYL DIKETOPIPERAZINE (UNII: XB09609XSL) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 9 in 1 BLISTER PACK 1 1 in 1 CARTRIDGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 07/11/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022472 11/09/2020 Labeler - Mannkind Corporation (099981040)
CloseEstablishment Name Address ID/FEI Business Operations Mannkind Corporation 099981040 MANUFACTURE(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) , ANALYSIS(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) , LABEL(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903) , PACK(47918-874, 47918-878, 47918-891, 47918-880, 47918-898, 47918-902, 47918-903)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
AFREZZA- insulin human powder, metered
AFREZZA- insulin human kit
Number of versions: 14
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Feb 29, 2024 | 14 (current) | download |
| Aug 16, 2023 | 13 | download |
| Apr 6, 2023 | 12 | download |
| Mar 23, 2021 | 11 | download |
| Feb 16, 2021 | 10 | download |
| Apr 3, 2020 | 9 | download |
| Oct 9, 2019 | 8 | download |
| Oct 29, 2018 | 7 | download |
| May 7, 2018 | 6 | download |
| Oct 10, 2017 | 5 | download |
| Jun 20, 2017 | 4 | download |
| Dec 13, 2016 | 3 | download |
| Aug 5, 2016 | 2 | download |
| Jun 28, 2016 | 1 | download |
RxNorm
AFREZZA- insulin human powder, metered
AFREZZA- insulin human kit
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1543202 | regular insulin, human 4 UNT Inhalation Powder Cartridge | PSN |
| 2 | 1543202 | insulin, regular, human 4 UNT Inhalation Powder | SCD |
| 3 | 1543202 | regular insulin, human 4 UNT Inhalation Powder Cartridge | SY |
| 4 | 1543207 | afrezza 4 UNT Inhalation Powder | PSN |
| 5 | 1543207 | insulin, regular, human 4 UNT Inhalation Powder [Afrezza] | SBD |
| 6 | 1543207 | Afrezza 4 UNT Inhalation Powder | SY |
| 7 | 1544488 | regular insulin, human 8 UNT Inhalation Powder Cartridge | PSN |
| 8 | 1544488 | insulin, regular, human 8 UNT Inhalation Powder | SCD |
| 9 | 1544488 | regular insulin, human 8 UNT Inhalation Powder Cartridge | SY |
| 10 | 1544490 | afrezza 8 UNT Inhalation Powder | PSN |
| 11 | 1544490 | insulin, regular, human 8 UNT Inhalation Powder [Afrezza] | SBD |
| 12 | 1544490 | Afrezza 8 UNT Inhalation Powder | SY |
| 13 | 1654910 | regular insulin, human 12 UNT Inhalation Powder Cartridge | PSN |
| 14 | 1654910 | insulin, regular, human 12 UNT Inhalation Powder | SCD |
| 15 | 1654910 | regular insulin, human 12 UNT Inhalation Powder Cartridge | SY |
| 16 | 1654912 | afrezza 12 UNT Inhalation Powder | PSN |
| 17 | 1654912 | insulin, regular, human 12 UNT Inhalation Powder [Afrezza] | SBD |
| 18 | 1654912 | Afrezza 12 UNT Inhalation Powder | SY |
| 19 | 1798387 | regular insulin, human 180 Inhalation Powder Titration Pack - 90 (4 UNT), 90 (8 UNT) | PSN |
| 20 | 1798387 | {90 (insulin, regular, human 4 UNT Inhalation Powder) / 90 (insulin, regular, human 8 UNT Inhalation Powder) } Pack | GPCK |
| 21 | 1798388 | afrezza 180 Inhalation Cartridge Titration Pack - 90 (4 UNT), 90 (8 UNT) | PSN |
| 22 | 1798388 | {90 (insulin, regular, human 4 UNT Inhalation Powder [Afrezza]) / 90 (insulin, regular, human 8 UNT Inhalation Powder [Afrezza]) } Pack [Afrezza Titration Pack] | BPCK |
| 23 | 1798388 | Afrezza Titration Pack- 90 (4 UNT), 90 (8 UNT) | SY |
| 24 | 1862101 | regular insulin, human 180 Inhalation Powder Pack - 60 (4 UNT), 60 (8 UNT), 60 (12 UNT) | PSN |
| 25 | 1862101 | {60 (insulin, regular, human 12 UNT Inhalation Powder) / 60 (insulin, regular, human 4 UNT Inhalation Powder) / 60 (insulin, regular, human 8 UNT Inhalation Powder) } Pack | GPCK |
| 26 | 1862101 | regular insulin, human 180 Inhalation Powder Pack - 60 (4 UNT), 60 (8 UNT), 60 (12 UNT) | SY |
| 27 | 1862102 | afrezza 180 Inhalation Cartridge Titration Pack - 60 (4 UNT), 60 (8 UNT), 60 (12 UNT) | PSN |
| 28 | 1862102 | {60 (insulin, regular, human 12 UNT Inhalation Powder [Afrezza]) / 60 (insulin, regular, human 4 UNT Inhalation Powder [Afrezza]) / 60 (insulin, regular, human 8 UNT Inhalation Powder [Afrezza]) } Pack [Afrezza 180 Titration Pack- 60 (4 UNT), 60 (8 UNT), 60 (12 UNT)] | BPCK |
| 29 | 1862102 | Afrezza 180 Inhalation Cartridge Titration Pack - 60 (4 UNT), 60 (8 UNT), 60 (12 UNT) | SY |
| 30 | 2100028 | regular insulin, human 180 Inhalation Cartridge Pack - 90 (8 UNT), 90 (12 UNT) | PSN |
| 31 | 2100028 | {90 (insulin, regular, human 12 UNT Inhalation Powder) / 90 (insulin, regular, human 8 UNT Inhalation Powder) } Pack | GPCK |
| 32 | 2100029 | afrezza 180 Inhalation Cartridge Pack - 90 (8 UNT), 90 (12 UNT) | PSN |
| 33 | 2100029 | {90 (insulin, regular, human 12 UNT Inhalation Powder [Afrezza]) / 90 (insulin, regular, human 8 UNT Inhalation Powder [Afrezza]) } Pack [Afrezza 180 - 90 (8 UNT), 90 (12 UNT)] | BPCK |
| 34 | 2100029 | afrezza 180 Cartridge Pack - 90 (8 UNT), 90 (12 UNT) | SY |
| 35 | 2644768 | insulin, regular, human 27 Inhalation Cartridge Sample Pack - 9 (4 UNT), 9 (8 UNT), 9 (12 UNT) | PSN |
| 36 | 2644768 | {9 (insulin, regular, human 12 UNT Inhalation Powder) / 9 (insulin, regular, human 4 UNT Inhalation Powder) / 9 (insulin, regular, human 8 UNT Inhalation Powder) } Pack | GPCK |
| 37 | 2644769 | afrezza 27 Inhalation Cartridge Sample Pack - 9 (4 UNT), 9 (8 UNT), 9 (12 UNT) | PSN |
| 38 | 2644769 | {9 (insulin, regular, human 12 UNT Inhalation Powder [Afrezza]) / 9 (insulin, regular, human 4 UNT Inhalation Powder [Afrezza]) / 9 (insulin, regular, human 8 UNT Inhalation Powder [Afrezza]) } Pack [Afrezza Sample Pack] | BPCK |
| 39 | 2644769 | Afrezza Sample Pack- 9 (4 UNT), 9 (8 UNT), 9 (12 UNT) | SY |
Get Label RSS Feed for this Drug
AFREZZA- insulin human powder, metered
AFREZZA- insulin human kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=29f4637b-e204-425b-b89c-7238008d8c10
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
AFREZZA- insulin human powder, metered
AFREZZA- insulin human kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 47918-874-90 |
| 2 | 47918-878-90 |
| 3 | 47918-880-18 |
| 4 | 47918-891-90 |
| 5 | 47918-898-18 |
| 6 | 47918-902-18 |
| 7 | 47918-903-27 |