Label: ADREVIEW- iobenguane i-123 injection
- NDC Code(s): 17156-235-01
- Packager: Medi-Physics Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AdreView safely and effectively. See full prescribing information for AdreView. AdreView (Iobenguane I 123 Injection) for ...
-
Table of ContentsTable of Contents
-
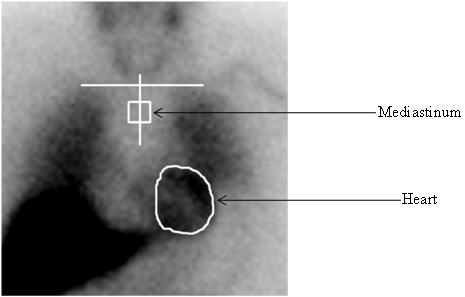
1 INDICATIONS AND USAGE1.1 Pheochromocytoma and Neuroblastoma - AdreView is a radiopharmaceutical indicated for use in the detection of primary or metastatic pheochromocytoma or neuroblastoma as an adjunct to other ...
-
2 DOSAGE AND ADMINISTRATION2.1 Radiation Safety - AdreView emits radiation and must be handled with appropriate safety measures to minimize radiation exposure to clinical personnel and patients. Radiopharmaceuticals should ...
-
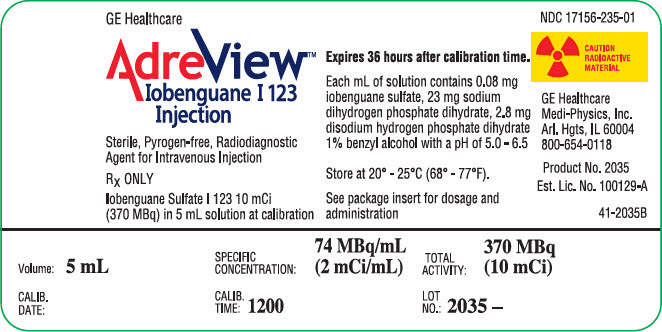
3 DOSAGE FORMS AND STRENGTHSSingle use vials containing 5 mL solution for intravenous injection (2 mCi/mL at calibration time).
-
4 CONTRAINDICATIONSAdreView is contraindicated in patients with known hypersensitivity to iobenguane or iobenguane sulfate.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions have been reported following AdreView administration. Prior to administration, question the patient for a history of prior reactions to ...
-
6 ADVERSE REACTIONS6.1 Clinical Study Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONSThe following drugs have the potential to decrease the uptake of norepinephrine and cause false negative imaging results: antihypertensives that deplete norepinephrine stores or inhibit reuptake ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Radioactive iodine products cross the placenta and can permanently impair fetal thyroid function. Administration of an appropriate thyroid blocking agent is ...
-
10 OVERDOSAGEThe major manifestations of overdose relate predominantly to increased radiation exposure, with the long term risks for neoplasia.
-
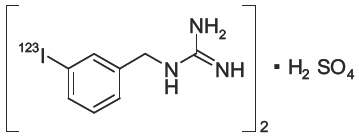
11 DESCRIPTIONAdreView (Iobenguane I 123 Injection) is a sterile, pyrogen-free radiopharmaceutical for intravenous injection. Each mL contains 0.08 mg iobenguane sulfate, 74 MBq (2 mCi) of I 123 (as iobenguane ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Iobenguane is similar in structure to the antihypertensive drug guanethedine and to the neurotransmitter norepinephrine (NE). Iobenguane is, therefore, largely subject ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Iobenguane hemisulfate was not mutagenic in vitro in the Ames bacterial mutation assay and in the in vitro mouse lymphoma test, and was ...
-
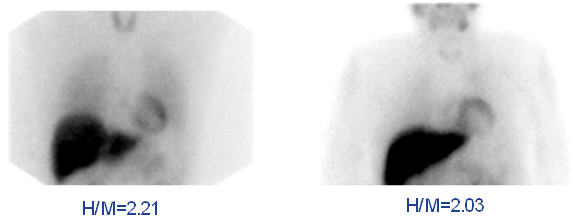
14 CLINICAL STUDIES14.1 Pheochromocytoma and Neuroblastoma - The safety and efficacy of AdreView were assessed in an open-label, multicenter, multinational trial of 251 subjects with known or suspected ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAdreView is supplied in 10 mL glass vials containing a total volume of 5 mL of solution with a total radioactivity of 370 MBq (10 mCi) at calibration time. Each vial is enclosed in a lead ...
-
17 PATIENT COUNSELING INFORMATIONInstruct patients to inform their physician or healthcare provider if they: are pregnant. Advise a pregnant woman of the potential risks of fetal exposure to radiation doses with AdreView [see ...
-
SPL UNCLASSIFIED SECTIONManufactured and Distributed by GE Healthcare, Medi-Physics, Inc., Arlington Heights, IL 60004 U.S.A. AdreView is a trademark of GE Healthcare or one of its subsidiaries. GE and the GE Monogram are ...
-
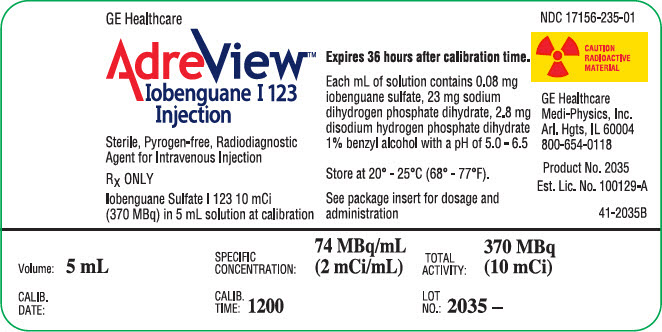
PRINCIPAL DISPLAY PANEL - 5 mL Vial LabelGE Healthcare - AdreView™ Iobenguane I 123 - Injection - Sterile, Pyrogen-free, Radiodiagnostic - Agent for Intravenous Injection - RX ONLY - Iobenguane Sulfate I 123 10 mCi - (370 MBq) in 5 mL solution at ...
-
INGREDIENTS AND APPEARANCEProduct Information