Label: ADDYI- flibanserin tablet, film coated
- NDC Code(s): 58604-214-03, 58604-214-30
- Packager: Sprout Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 13, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADDYI safely and effectively. See full prescribing information for ADDYI. ADDYI (flibanserin) tablets, for oral use - Initial U.S ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPOTENSION AND SYNCOPE IN CERTAIN SETTINGS
Interaction with Alcohol
The use of ADDYI and alcohol together close in time increases the risk of severe hypotension and syncope [see Warnings and Precautions (5.1)]. Counsel patients to wait at least two hours after consuming one or two standard alcoholic drinks before taking ADDYI at bedtime or to skip their ADDYI dose if they have consumed three or more standard alcoholic drinks that evening.
Contraindicated with Strong or Moderate CYP3A4 Inhibitors
The concomitant use of ADDYI and moderate or strong CYP3A4 inhibitors increases flibanserin concentrations, which can cause severe hypotension and syncope [see Warnings and Precautions (5.2)]. Therefore, the use of moderate or strong CYP3A4 inhibitors is contraindicated in patients taking ADDYI [see Contraindications (4)].
Contraindicated in Patients with Hepatic Impairment
The use of ADDYI in patients with hepatic impairment increases flibanserin concentrations, which can cause severe hypotension and syncope [see Warnings and Precautions (5.5)]. Therefore, ADDYI is contraindicated in patients with hepatic impairment [see Contraindications (4)].
Close -
1 INDICATIONS AND USAGEADDYI is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder (HSDD), as characterized by low sexual desire that causes marked distress ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of ADDYI is 100 mg administered orally once per day at bedtime. ADDYI is dosed at bedtime because administration during waking hours increases the ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 100 mg, oval, pink, debossed on one side with "f100" and blank on the other side.
-
4 CONTRAINDICATIONSADDYI is contraindicated in patients: • Using concomitant moderate or strong CYP3A4 inhibitors [see Boxed Warning and Warnings and Precautions (5.2)]. • With hepatic impairment [see Boxed ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension and Syncope due to an Interaction with Alcohol - Taking ADDYI within two hours after consuming alcohol increases the risk of severe hypotension and syncope. To reduce this risk ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: • Hypotension and syncope [see Warnings and Precautions (5.1, 5.2, 5.4, 5.5)] • CNS depression ...
-
7 DRUG INTERACTIONSTable 3 contains clinically significant drug interactions (DI) with ADDYI. Table 3: Clinically Significant Drug Interactions with ADDYI - Alcohol - Clinical Implications - The ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no studies of ADDYI in pregnant women to inform whether there is a drug-associated risk in humans. In animals, fetal toxicity only occurred in the ...
-
10 OVERDOSAGEOverdosage of ADDYI may cause an increase in the incidence or severity of any of the reported adverse reactions [see Warnings and Precautions (5.3, 5.4) and Adverse Reactions (6.1)]. In the event ...
-
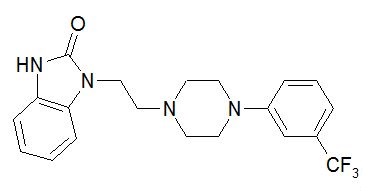
11 DESCRIPTIONADDYI (flibanserin) is a tablet for oral administration. The chemical name of flibanserin is 2H-Benzimidazol-2-one, 1,3-dihydro-1-[2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethyl]. Its ...
-
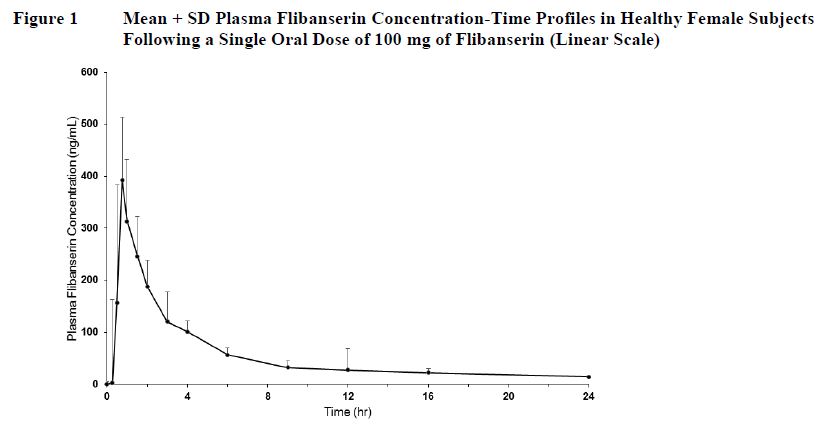
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of ADDYI in the treatment of premenopausal women with hypoactive sexual desire disorder is not known. 12.2 Pharmacodynamics - Receptor ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - A two-year carcinogenicity study was conducted in CD-1 mice with dietary administration of 0, 10, 80, 200 and ...
-
14 CLINICAL STUDIES14.1 Studies in Premenopausal HSDD Patients - The efficacy of ADDYI for the treatment of HSDD in premenopausal women was established in three 24-week, randomized, double-blind, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGADDYI is available as a 100 mg oval, pink, film-coated tablet debossed on one side with “f100” and blank on the other side. Available in bottles of 30 tablets. (NDC 58604-214-30) Storage - Store ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (Medication Guide). Hypotension and Syncope - Inform patients that ADDYI can cause severe hypotension and syncope, particularly when taken close in time with ...
-
MEDICATION GUIDEADDYI® (add-ee) (flibanserin) tablets, for oral use - Read this Medication Guide before you start taking ADDYI® and each time you get a refill. There may be new information. This information ...
-
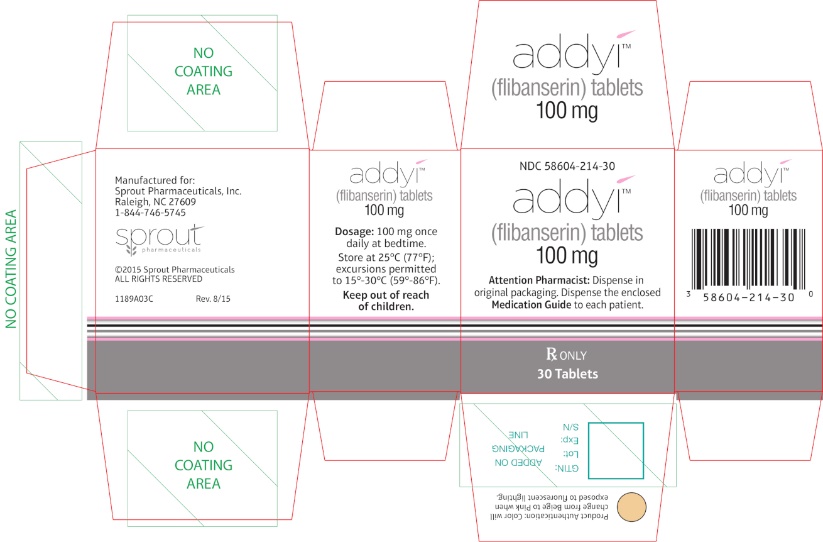
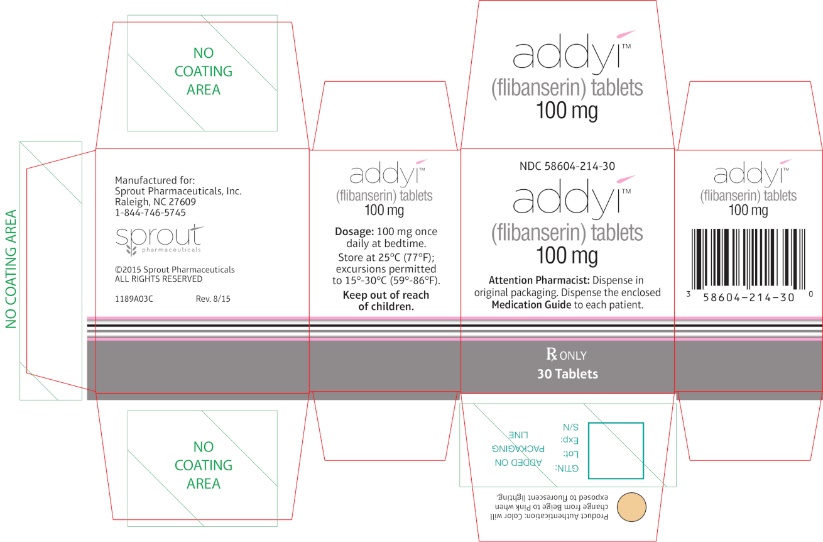
PRINCIPAL DISPLAY PANEL - Carton LabelRx only - NDC 58604-214-30 - addyi® (flibanserin) tablets - 100 mg - Attention Pharmacist: Dispense in original packaging. Dispense the accompanying Medication Guide to each patient. 30 ...
-
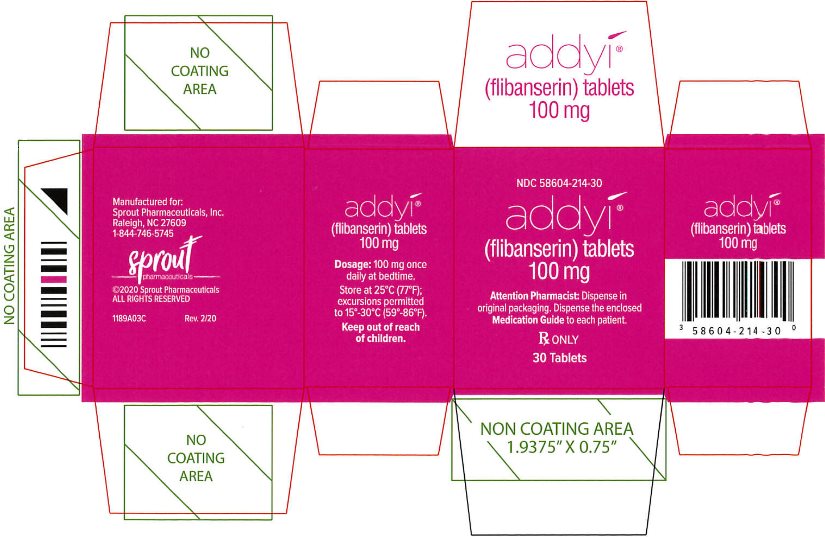
PRINCIPAL DISPLAY PANEL - Carton Label (New)
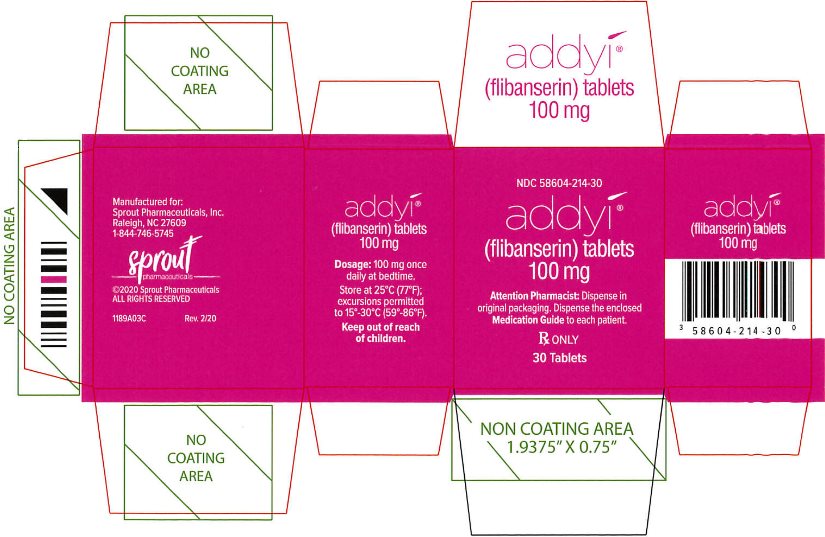
-
PRINCIPAL DISPLAY PANEL - Bottle LabelRx only - NDC 58604-214-30 - addyi® (flibanserin) tablets - 100 mg - Attention Pharmacist: Dispense in original packaging. Dispense the accompanying Medication Guide to each patient. 30 ...
-
PRINCIPAL DISPLAY PANEL – Bottle Label (New)

-
INGREDIENTS AND APPEARANCEProduct Information