Label: ADASUVE- loxapine aerosol, powder
- NDC Code(s): 51097-001-01, 51097-001-02
- Packager: Alexza Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADASUVE safely and effectively. See full prescribing information for ADASUVE. ADASUVE - ® (loxapine) inhalation powder, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)Bronchospasm - ADASUVE can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest, particularly in patients with lung diseases. Administer ADASUVE only in ...
WARNING: BRONCHOSPASM and INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Bronchospasm
ADASUVE can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest, particularly in patients with lung diseases. Administer ADASUVE only in a certified healthcare setting that has immediate access on site to supplies and healthcare professionals competent in the management of acute bronchospasm and access to emergency assistance for symptoms that require immediate medical attention [see Warnings and Precautions (5.1, 5.2)] . Certified healthcare settings must have a short-acting bronchodilator (e.g. albuterol) available for the immediate treatment of bronchospasm; this short-acting bronchodilator can be delivered by inhaler (with spacer) or nebulizer. Prior to administering ADASUVE, screen patients regarding a current diagnosis, history, or symptoms of asthma, COPD and other lung diseases, and assess (including chest auscultation) patients for respiratory signs. Monitor for signs and symptoms of bronchospasm following treatment with ADASUVE [see Dosage and Administration (2.2, 2.4) and Contraindications (4)] .
Because of the risk of bronchospasm, ADASUVE is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ADASUVE REMS [see Warnings and Precautions (5.2)] .
CloseIncreased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ADASUVE is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.3)] .
-
1 INDICATIONS AND USAGEADASUVE is indicated for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults - [see - Clinical Studies (14)] ...
-
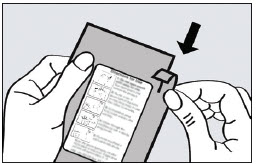
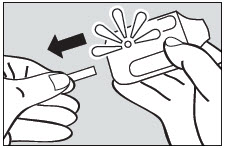
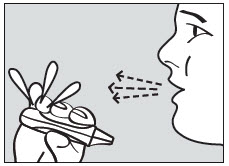
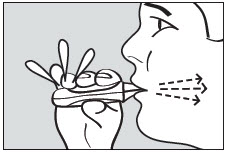
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - ADASUVE must be administered only by a healthcare professional. ADASUVE is administered by oral inhalation only. The recommended dose for acute agitation is 10 mg ...
-
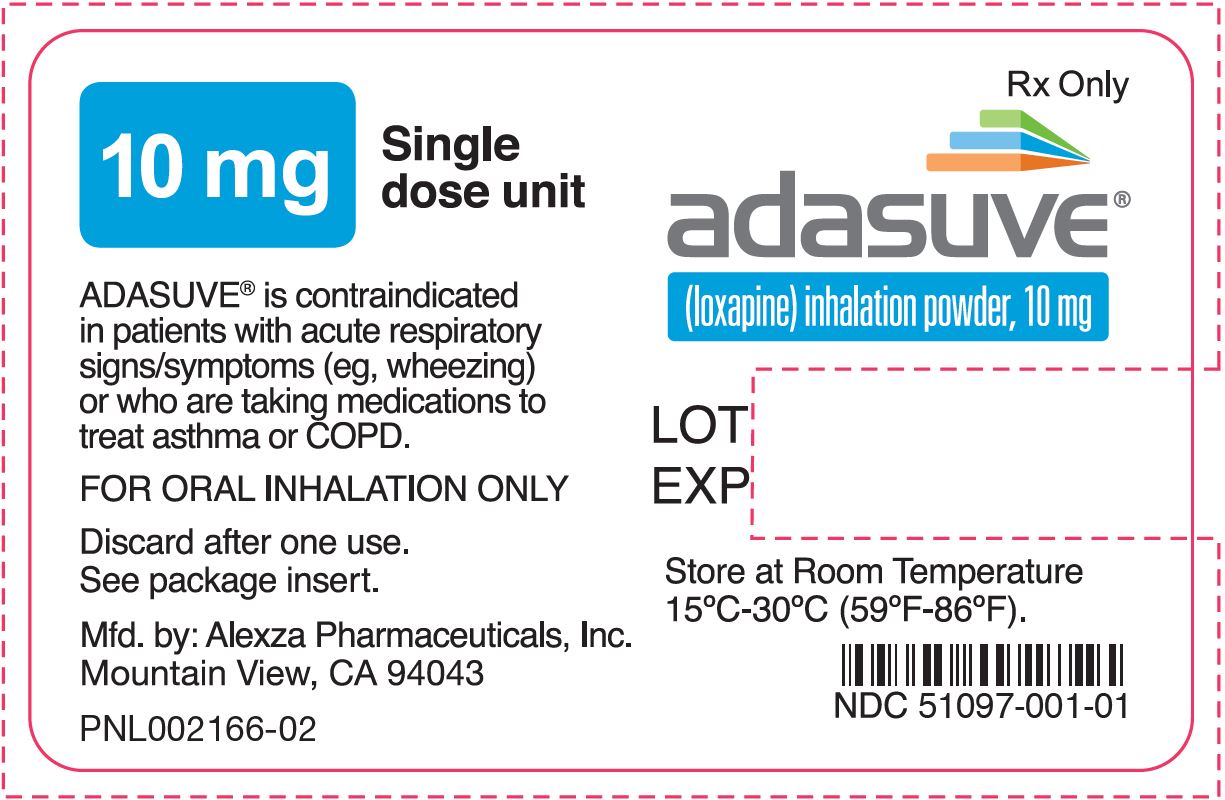
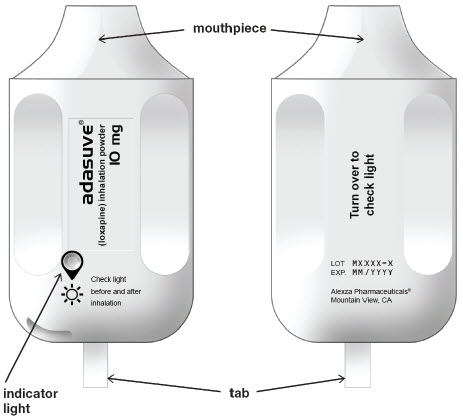
3 DOSAGE FORMS AND STRENGTHSADASUVE is an inhalation powder supplied in a single-use, disposable inhaler containing 10 mg of loxapine base.
-
4 CONTRAINDICATIONSADASUVE is contraindicated in patients with the following: Current diagnosis or history of asthma, COPD, or other lung disease associated with bronchospasm - [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Bronchospasm - ADASUVE can cause bronchospasm that has the potential to lead to respiratory distress and respiratory arrest - [see - Adverse Reactions ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in more detail in other sections of the labeling: Hypersensitivity (serious skin reactions) [see - Contraindications ...
-
7 DRUG INTERACTIONS7.1 CNS Depressants - ADASUVE is a central nervous system (CNS) depressant. The concurrent use of ADASUVE with other CNS depressants (e.g., alcohol, opioid analgesics, benzodiazepines, tricyclic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics during pregnancy. Healthcare ...
-
10 OVERDOSAGESigns and Symptoms of Overdosage - As would be expected from the pharmacologic actions of loxapine, the clinical findings may include CNS depression, unconsciousness, profound hypotension ...
-
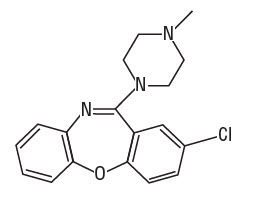
11 DESCRIPTIONADASUVE, an atypical antipsychotic, is an inhalation powder of loxapine supplied in a single-use, disposable inhaler containing 10 mg of loxapine base. ADASUVE is a drug-device combination ...
-
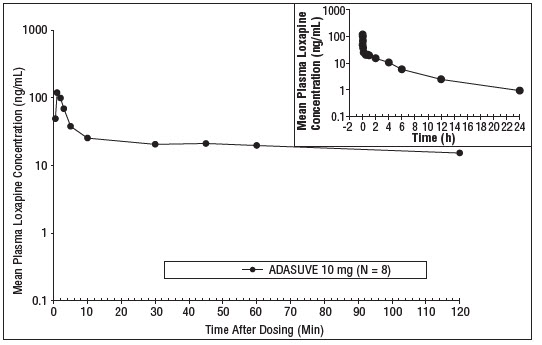
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of loxapine in the treatment of agitation associated with schizophrenia and bipolar I disorder is unclear. However, its efficacy could be ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: No studies have been conducted. Mutagenesis: Loxapine was not mutagenic in the in vitro ...
-
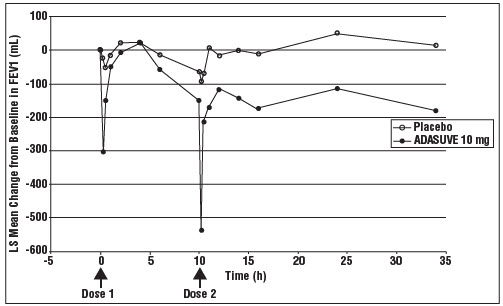
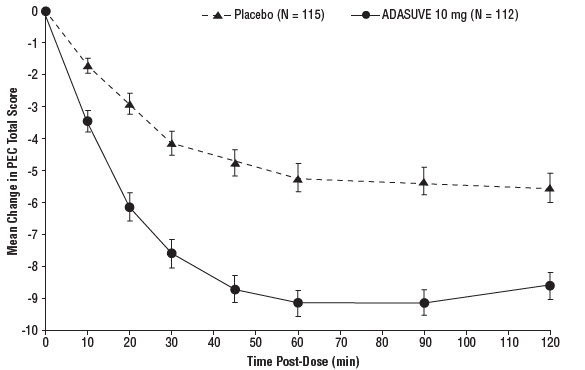
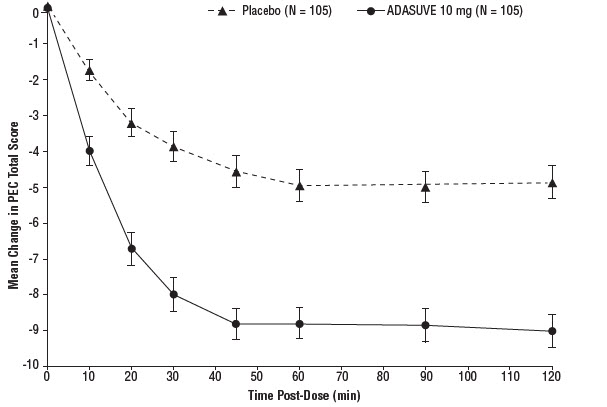
14 CLINICAL STUDIESThe efficacy of ADASUVE 10 mg in the acute treatment of agitation associated with schizophrenia or bipolar I disorder was established in two short-term (24-hour), randomized, double-blind ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ADASUVE - ® (loxapine) inhalation powder is supplied as: ADASUVE 10 mg (NDC 51097-001-01) is a single-use, disposable inhaler containing 10 mg of loxapine ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Bronchospasm - Advise patients and caregivers that there is a risk of bronchospasm. Advise patients to inform ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Alexza Pharmaceuticals, Inc., Mountain View, CA 94043
-
MEDICATION GUIDE
ADASUVE
® (AD-uh-soov)
(loxapine)
Inhalation Powder
Read this Medication Guide before you start taking ADASUVE and each time it is given to you. There may be new information. This Medication Guide does not take the place of talking to your ...
-
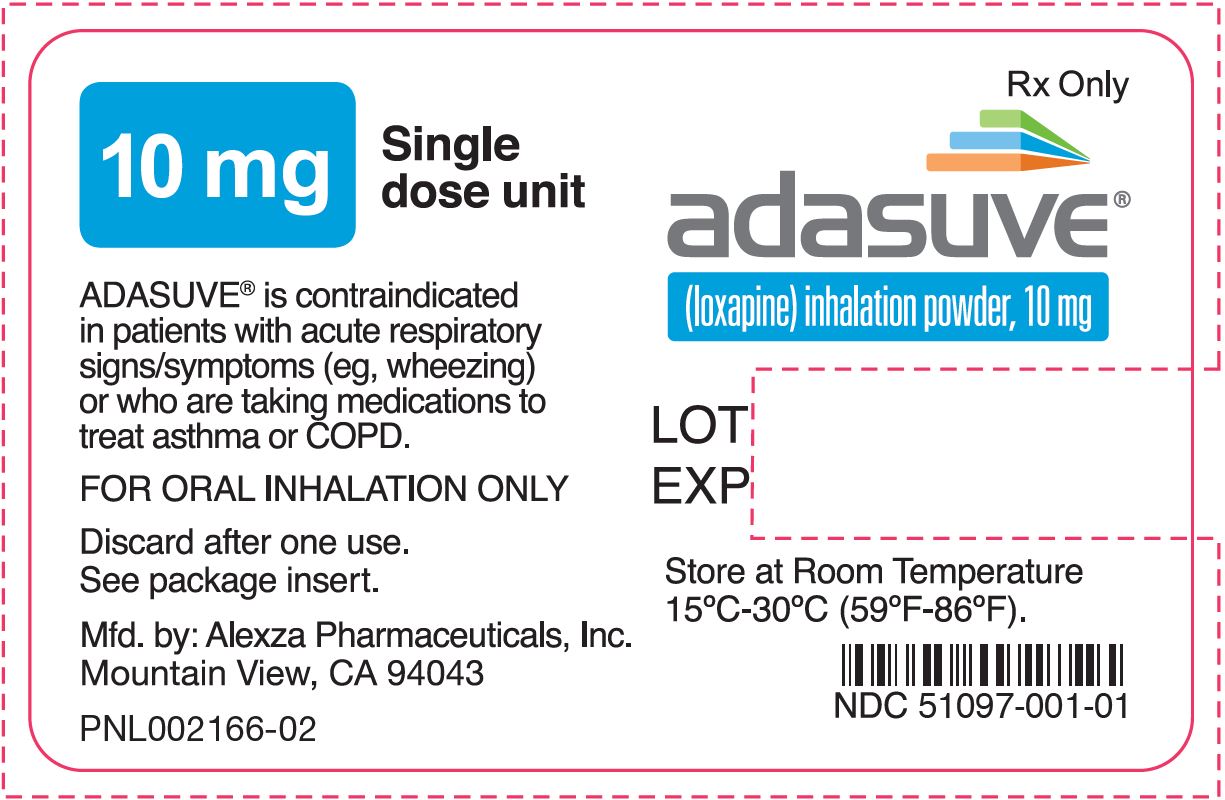
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 51097-001-01 - FOR ORAL INHALATION ONLY - adasuve - ™ (loxapine) inhalation powder - 10 mg - 5 single dose units per carton - R - x ONLY ...
-
INGREDIENTS AND APPEARANCEProduct Information