Label: ADAKVEO- crizanlizumab injection
- NDC Code(s): 0078-0883-61
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADAKVEO safely and effectively. See full prescribing information for ADAKVEO. ADAKVEO® (crizanlizumab-tmca) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEADAKVEO® is indicated to reduce the frequency of vaso-occlusive crises (VOCs) in adults and pediatric patients aged 16 years and older with sickle cell disease.
-
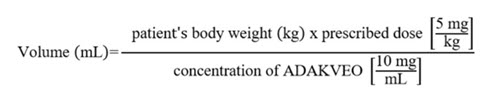
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Administer ADAKVEO 5 mg/kg by intravenous infusion over a period of 30 minutes at Week 0, Week 2, and every 4 weeks thereafter. If a dose is missed, administer ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 100 mg/10 mL (10 mg/mL) as a clear to opalescent, colorless to slightly brownish-yellow solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Infusion-Related Reactions - In the SUSTAIN clinical trial, infusion-related reactions (IRRs) (defined as occurring during/within 24 hours of infusion) were observed in 2 (3%) patients ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Infusion-related reactions [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Laboratory Test Interference - Platelet Tests - ADAKVEO interferes with automated platelet counts (platelet clumping) in particular when blood samples are collected in tubes containing ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on data from animal studies, ADAKVEO has the potential to cause fetal harm when administered to a pregnant woman. In an animal reproduction study ...
-
11 DESCRIPTIONCrizanlizumab-tmca is a P-selectin blocker humanized IgG2 kappa monoclonal antibody that binds to P-selectin. Crizanlizumab-tmca is produced using recombinant DNA technology in Chinese hamster ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Crizanlizumab-tmca is a humanized IgG2 kappa monoclonal antibody that binds to P-selectin and blocks interactions with its ligands, including P-selectin ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with crizanlizumab-tmca. In the 26-week repeat-dose toxicity study ...
-
14 CLINICAL STUDIESSUSTAIN - The efficacy of ADAKVEO was evaluated in patients with sickle cell disease in SUSTAIN [NCT01895361], a 52-week, randomized, multicenter, placebo-controlled, double-blind study. A total ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ADAKVEO (crizanlizumab-tmca) injection is a sterile, clear to opalescent, colorless to slightly brownish-yellow solution for intravenous infusion supplied as: Carton containing one ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Infusion-Related Reactions - Advise patients to contact their healthcare provider immediately for signs or ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 6/2024 - PATIENT INFORMATION - ADAKVEO® (ah dak vee oh) (crizanlizumab-tmca) injection, for ...
-
PRINCIPAL DISPLAY PANEL
NDC 0078-0883-61 - Rx Only - ADAKVEO® (crizanlizumab-tmca) Injection - 100 mg/10 mL - (10 mg/mL) For intravenous infusion after dilution. 1 Single-Dose Vial. Discard Unused Portion. NOVARTIS
-
INGREDIENTS AND APPEARANCEProduct Information