Label: ACUVAIL- ketorolac tromethamine solution/ drops
- NDC Code(s): 0023-3507-05, 0023-3507-30, 0023-3507-31
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ACUVAIL safely and effectively. See full prescribing information for ACUVAIL. ACUVAIL® (ketorolac tromethamine ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
ACUVAIL® is indicated for the treatment of pain and inflammation following cataract surgery.
-
2
DOSAGE AND ADMINISTRATION
2.1 - Recommended Dosage - Apply one drop of ACUVAIL to the affected eye twice daily beginning 1 day prior to cataract surgery, continued on the day of surgery, and through the first 2 ...
-
3
DOSAGE FORMS AND STRENGTHS
ACUVAIL (ketorolac tromethamine ophthalmic solution) is a sterile, clear, and colorless to pale yellow ophthalmic solution containing 0.45% (4.5 mg/mL) ketorolac tromethamine in a ...
-
4
CONTRAINDICATIONS
ACUVAIL is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation [see Adverse Reactions (6.1)].
-
5
WARNINGS AND PRECAUTIONS
5.1 - Delayed Healing - Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use ...
-
6
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Delayed Healing [see Warnings and Precautions (5.1)] Cross-Sensitivity or Hypersensitivity [see Warnings and ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no adequate and well-controlled studies with ACUVAIL in pregnant women. No evidence of teratogenicity has been observed in rats or rabbits with ...
-
11
DESCRIPTION
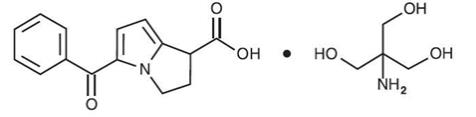
ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is a member of the pyrrolo-pyrrole group of nonsteroidal anti-inflammatory drugs (NSAIDs) for topical ophthalmic use. Its chemical name ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Ketorolac tromethamine is a nonsteroidal anti-inflammatory drug which, when administered systemically, has demonstrated analgesic, anti-inflammatory, and ...
-
14
CLINICAL STUDIES
Two multicenter, randomized, double-masked, parallel group comparison studies including approximately 500 patients were conducted to evaluate the effects of ACUVAIL on anterior chamber cell and ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
ACUVAIL (ketorolac tromethamine ophthalmic solution) 0.45% is supplied as a sterile, clear, and colorless to pale yellow ophthalmic solution in clear, low density polyethylene (LDPE), single-dose ...
-
17
PATIENT COUNSELING INFORMATION
Slow or Delayed Healing - Advise patients of the possibility that slow or delayed healing may occur while using nonsteroidal anti-inflammatory drugs (NSAIDs). Avoiding Contamination of the ...
-
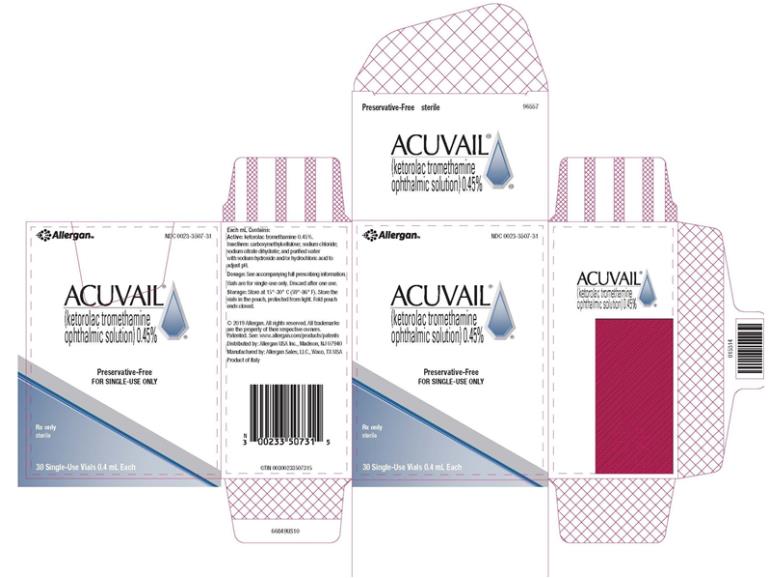
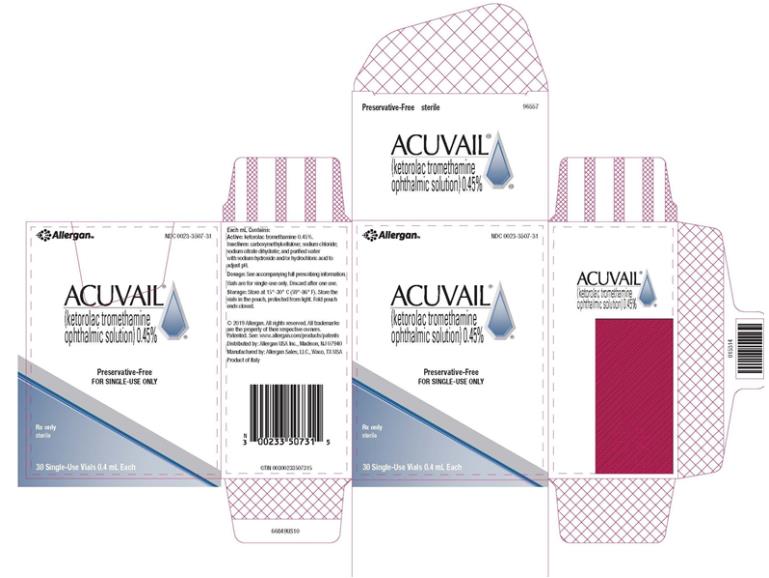
PRINCIPAL DISPLAY PANEL
NDC 0023-3507-31 - abbvie - ACUVAIL® (ketorolac tromethamine - ophthalmic solution)0.45% FOT TOPICAL OPHTHALMIC USE - Contains No Anti-Microbial Preservatives - FOR SINGLE-DOSE ONLY - Rx ...
-
PRINCIPAL DISPLAY PANEL
NDC 0023-3507-30
-
INGREDIENTS AND APPEARANCEProduct Information