Label: ACTIMMUNE- interferon gamma-1b injection, solution
- NDC Code(s): 75987-111-10, 75987-111-11
- Packager: Horizon Therapeutics USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 12, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ACTIMMUNE® safely and effectively. See full prescribing information for ACTIMMUNE. ACTIMMUNE® (interferon gamma-1b) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEACTIMMUNE is indicated for reducing the frequency and severity of serious infections associated with Chronic Granulomatous Disease (CGD). ACTIMMUNE is indicated for delaying time to disease ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dosage of ACTIMMUNE administered subcutaneously, for the treatment of patients with CGD and SMO is shown in Table 1 below: Table 1: Recommended ...
-
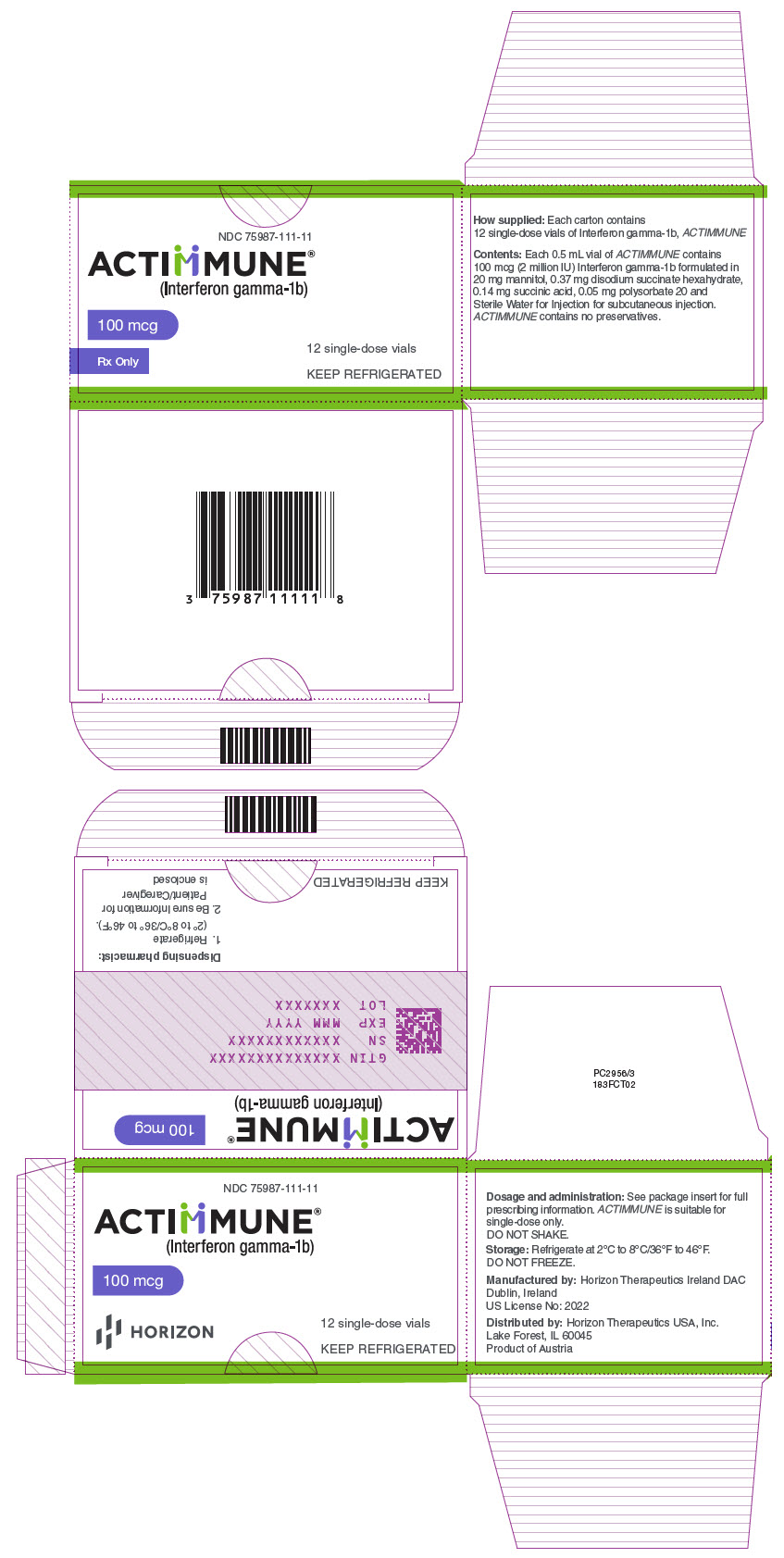
3 DOSAGE FORMS AND STRENGTHSInjection: 100 mcg (2 million International Units) per 0.5 mL solution in a single-dose vial. ACTIMMUNE (interferon gamma-1b) is a sterile, clear, colorless solution filled in a single-dose vial ...
-
4 CONTRAINDICATIONSACTIMMUNE is contraindicated in patients who develop or have known hypersensitivity to interferon gamma, E. coli derived products, or any component of the product.
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Disorders - Acute and transient "flu-like" symptoms such as fever and chills induced by ACTIMMUNE at doses of 250 mcg/m2/day (greater than 10 times the weekly recommended dose ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described below and elsewhere in the warnings and precautions section of the labeling: Cardiovascular Disorders [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Myelosuppressive Agents - When administering ACTIMMUNE in combination with other potentially myelosuppressive agents, monitor neutrophil and platelet counts [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies in pregnant women. ACTIMMUNE should be used during pregnancy only if the potential benefit justifies the ...
-
10 OVERDOSAGECentral nervous system adverse reactions including decreased mental status, gait disturbance and dizziness have been observed, particularly in patients receiving doses greater than 100 mcg/m2/day ...
-
11 DESCRIPTIONACTIMMUNE (Interferon gamma-1b), an interferon gamma, is a single-chain polypeptide containing 140 amino acids. Production of ACTIMMUNE is achieved by fermentation of a genetically engineered ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Interferons bind to specific cell surface receptors and initiate a sequence of intracellular events that lead to the transcription of interferon-stimulated genes. The ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis: ACTIMMUNE has not been tested for its carcinogenic potential. Mutagenesis: Ames tests using five different ...
-
14 CLINICAL STUDIES14.1 Effects in Chronic Granulomatous Disease (CGD) A randomized, double-blind, placebo-controlled trial of ACTIMMUNE (interferon gamma-1b) in patients with CGD, was performed to determine ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ACTIMMUNE (interferon gamma-1b) is a sterile, clear, colorless solution filled in a single-dose vial for subcutaneous injection. Each vial permits the extraction of up to 0.5 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or their parents or caregivers to read the FDA-approved patient labeling (Information for Patient/Caregiver). Inform patients and/or their parents or caregiver regarding ...
-
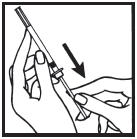
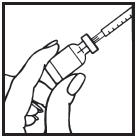
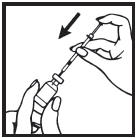
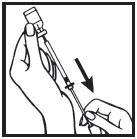
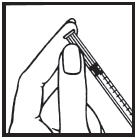
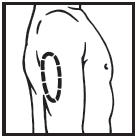
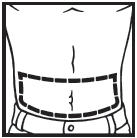
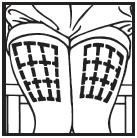
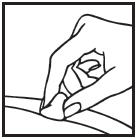
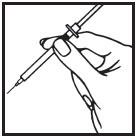
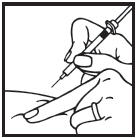
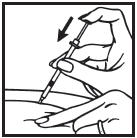
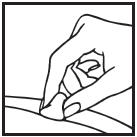
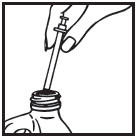
Information for the Patient/Caregiver ACTIMMUNE® (Interferon gamma-1b)DO NOT ADMINISTER ACTIMMUNE UNTIL YOUR PHYSICIAN HAS THOROUGHLY TRAINED YOU IN THE PROPER TECHNIQUES. ACTIMMUNE is supplied in single-dose vials. The unused portion of each vial should be ...
-
STORAGEACTIMMUNE (Interferon gamma-1b) must be refrigerated immediately. Refrigerate at 36° to 46° Fahrenheit (2° to 8° Centigrade). DO NOT FREEZE. ACTIMMUNE is supplied in single- dose vials. The ...
-
SPL UNCLASSIFIED SECTIONDecember 2019 - Manufactured by: Horizon Therapeutics Ireland DAC - Dublin, Ireland - U.S. License No. 2022 - Distributed by: Horizon Therapeutics USA, Inc. Lake Forest, IL 60045 - ACT-US-IFU-001
-
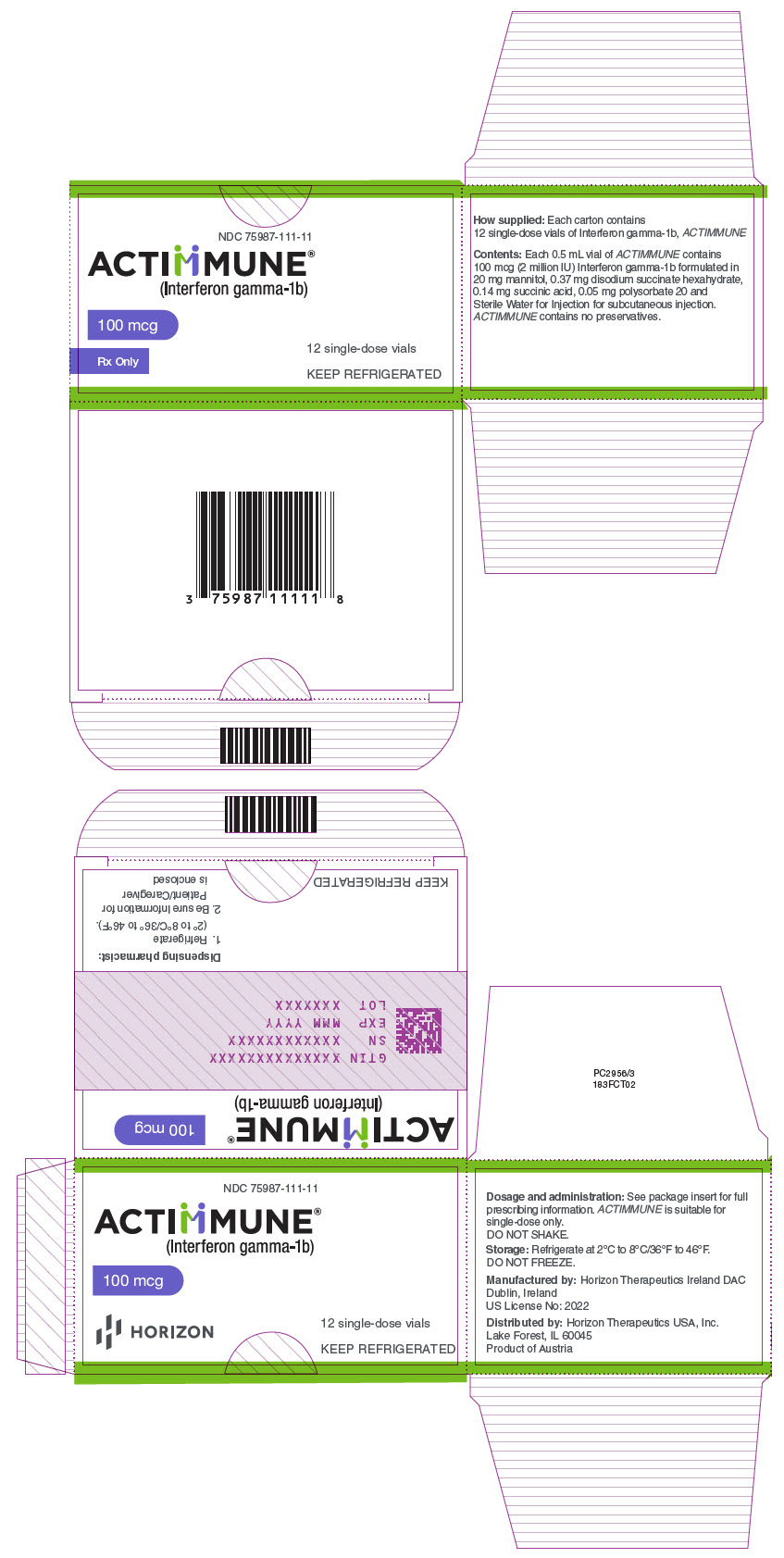
PRINCIPAL DISPLAY PANEL - 100 mcg Vial CartonNDC 75987-111-11 - ACTIMMUNE® (Interferon gamma-1b) 100 mcg - Rx Only - 12 single-dose vials - KEEP REFRIGERATED
-
INGREDIENTS AND APPEARANCEProduct Information