Label: ACTHREL- corticorelin ovine triflutate injection, powder, lyophilized, for solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 55566-0302-1 - Packager: Ferring Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 12, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor intravenous injection only - DIAGNOSTIC USE ONLY
-
DESCRIPTIONACTHREL® (corticorelin ovine triflutate for injection) is a sterile, nonpyrogenic, lyophilized white cake powder, containing corticorelin ovine triflutate, a trifluoroacetate salt of a synthetic ...
-
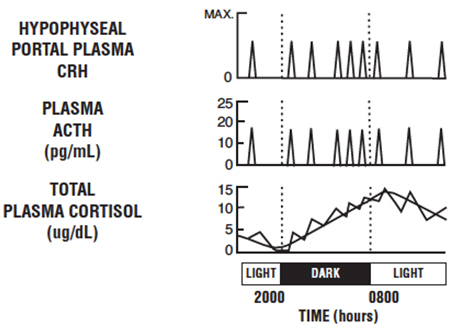
CLINICAL PHARMACOLOGYPharmacodynamics - In normal subjects, intravenous administration of corticorelin results in a rapid and sustained increase of plasma ACTH levels and a near parallel increase of plasma cortisol ...
-
INDICATIONS AND USAGEACTHREL® is indicated for use in differentiating pituitary and ectopic production of ACTH in patients with ACTH-dependent Cushing's syndrome. Differential Diagnosis - There are two forms of ...
-
CONTRAINDICATIONSACTHREL is contraindicated in patients with a history of a hypersensitivity reaction to ovine corticorelin or any of its excipients.
-
PRECAUTIONSGeneral - The severity of adverse effects to a corticorelin injection appear to be dose-dependent. Dosages above 1 mcg/kg are not recommended. While few adverse effects have been observed at the ...
-
ADVERSE REACTIONSHypersensitivity reactions have been reported with 1 mcg/kg or 100 mcg/patient and include flushing of the face, neck, and upper chest; dyspnea, wheezing, urticaria, and angioedema (involving ...
-
OVERDOSAGESymptoms of overdose include severe facial flushing, cardiovascular changes, and dyspnea. In the event of toxic overdose (see ADVERSE REACTIONS), adverse effects should be treated ...
-
DOSAGE AND ADMINISTRATIONDosage - A single intravenous dose of ACTHREL® at 1 mcg/kg is recommended for the testing of pituitary corticotrophin function. A dose of 1 mcg/kg is the lowest dose that produces maximal ...
-
HOW SUPPLIEDACTHREL® is supplied as a sterile, nonpyrogenic, lyophilized, white cake containing 100 mcg corticorelin ovine (as the trifluoroacetate), 0.88 mg ascorbic acid, 10 mg lactose, and 26 mg cysteine ...
-
SPL UNCLASSIFIED SECTIONMANUFACTURED FOR: FERRING PHARMACEUTICALS INC. PARSIPPANY, NJ 07054 - Origin Germany - Rx only - Rev. 09/2018 - XXXXXXXXXX
-
PRINCIPAL DISPLAY PANEL - 100 mcg Vial Label100 mcg - ACTHREL® (corticorelin ovine triflutate for injection) NDC 55566-0302-1 - Contains 100 mcg corticorelin ovine (as the trifluoroacetate), 10 mg lactose, 0.88 mg ascorbic acid, and 26 ...
-
INGREDIENTS AND APPEARANCEProduct Information