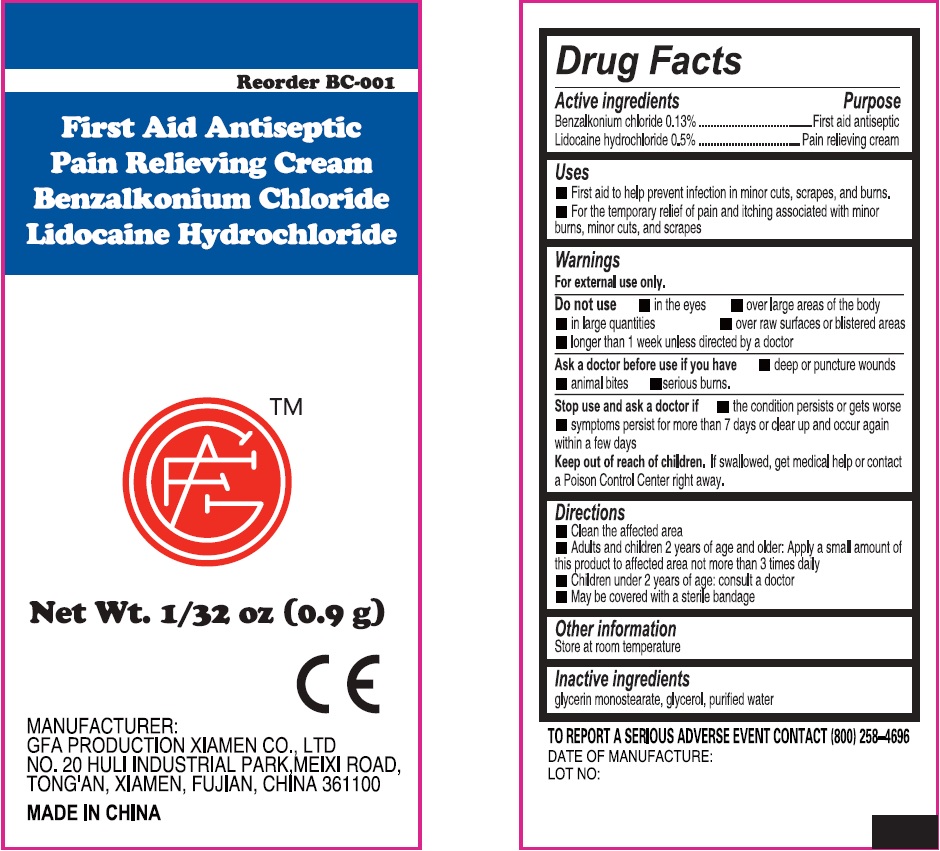
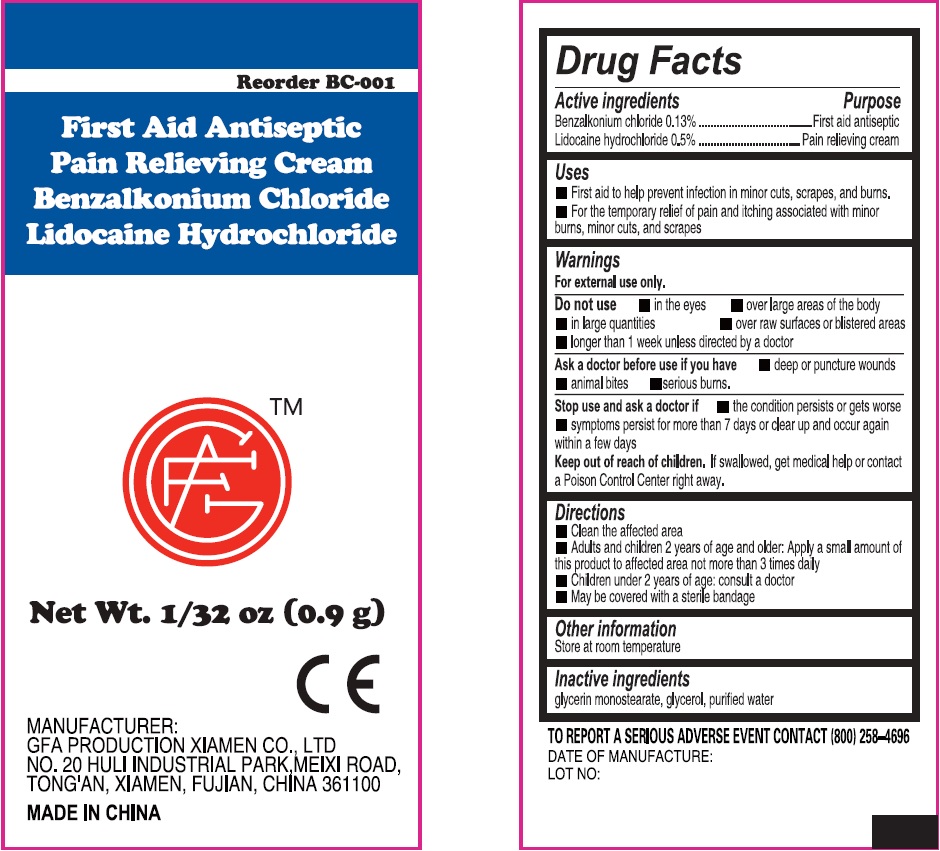
Label: FIRST AID ANTISEPTIC PAIN RELIEVING- benzalkonium chloride, lidocaine hydrochloride cream
- NDC Code(s): 50814-009-01
- Packager: GFA Production (Xiamen) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
-
Warnings
For external use only.
Do not use
• in the eyes • over large areas of the body • in large quantities • over raw surfaces or blistered areas • longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
FIRST AID ANTISEPTIC PAIN RELIEVING
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50814-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-009-01 0.9 g in 1 PACKAGE; Type 0: Not a Combination Product 07/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 07/01/2016 Labeler - GFA Production (Xiamen) Co., Ltd. (421256261)