Label: PRINCIPAL SECRET ADVANCED WITH HYDROSPHERES CONTINUOUS MOISTURE SPF8- octinoxate and octisalate cream
- NDC Code(s): 70605-017-34
- Packager: Guthy-Renker LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
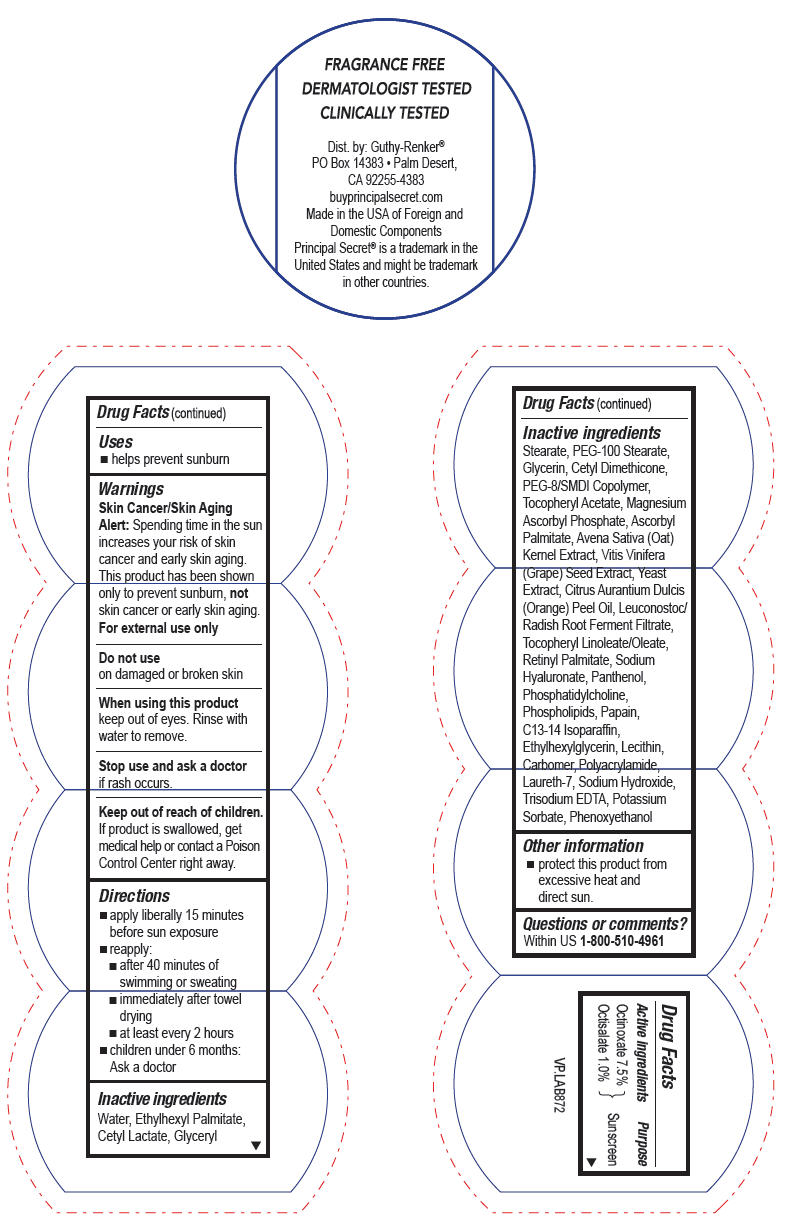
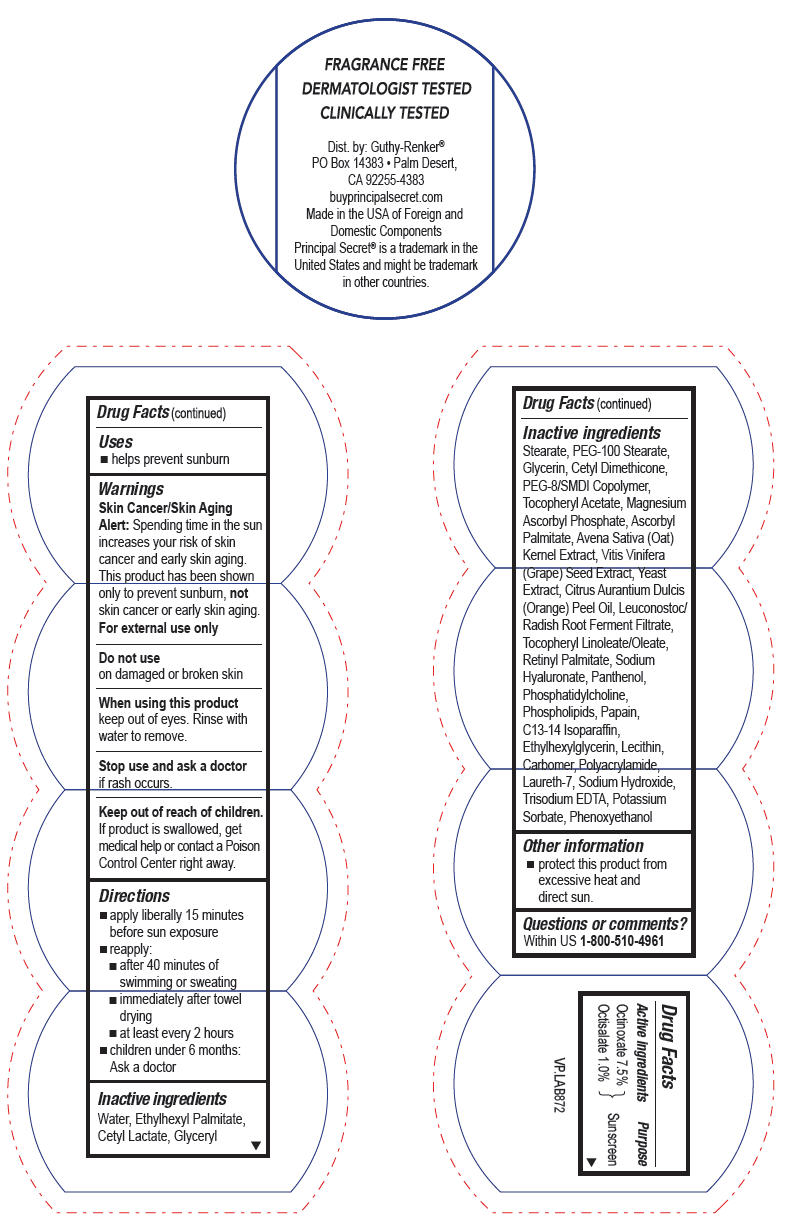
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Water, Ethylhexyl Palmitate, Cetyl Lactate, Glyceryl Stearate, PEG-100 Stearate, Glycerin, Cetyl Dimethicone, PEG-8/SMDI Copolymer, Tocopheryl Acetate, Magnesium Ascorbyl Phosphate, Ascorbyl Palmitate, Avena Sativa (Oat) Kernel Extract, Vitis Vinifera (Grape) Seed Extract, Yeast Extract, Citrus Aurantium Dulcis (Orange) Peel Oil, Leuconostoc/Radish Root Ferment Filtrate, Tocopheryl Linoleate/Oleate, Retinyl Palmitate, Sodium Hyaluronate, Panthenol, Phosphatidylcholine, Phospholipids, Papain, C13-14 Isoparaffin, Ethylhexylglycerin, Lecithin, Carbomer, Polyacrylamide, Laureth-7, Sodium Hydroxide, Trisodium EDTA, Potassium Sorbate, Phenoxyethanol
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 57 g Jar Label
-
INGREDIENTS AND APPEARANCE
PRINCIPAL SECRET ADVANCED WITH HYDROSPHERES CONTINUOUS MOISTURE SPF8
octinoxate and octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70605-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.5 g in 100 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 1 g in 100 g Inactive Ingredients Ingredient Name Strength ETHYLHEXYL PALMITATE (UNII: 2865993309) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) Glycerin (UNII: PDC6A3C0OX) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) PANTHENOL (UNII: WV9CM0O67Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) WINE GRAPE (UNII: 3GOV20705G) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) ASCORBYL PALMITATE (UNII: QN83US2B0N) PANTOTHENIC ACID (UNII: 19F5HK2737) PHYTONADIONE (UNII: A034SE7857) BERGAMOT OIL (UNII: 39W1PKE3JI) LIMONENE, (+)- (UNII: GFD7C86Q1W) AVENA SATIVA FLOWERING TOP (UNII: MA9CQJ3F7F) PAPAIN (UNII: A236A06Y32) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) LAURETH-7 (UNII: Z95S6G8201) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) PEG-8 LAURATE (UNII: 762O8IWA10) TROLAMINE (UNII: 9O3K93S3TK) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70605-017-34 57 g in 1 JAR; Type 0: Not a Combination Product 12/31/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 12/31/2017 Labeler - Guthy-Renker LLC (948861877) Establishment Name Address ID/FEI Business Operations Universal Packaging Systems, Inc. (DBA Paklab) 177711082 MANUFACTURE(70605-017)