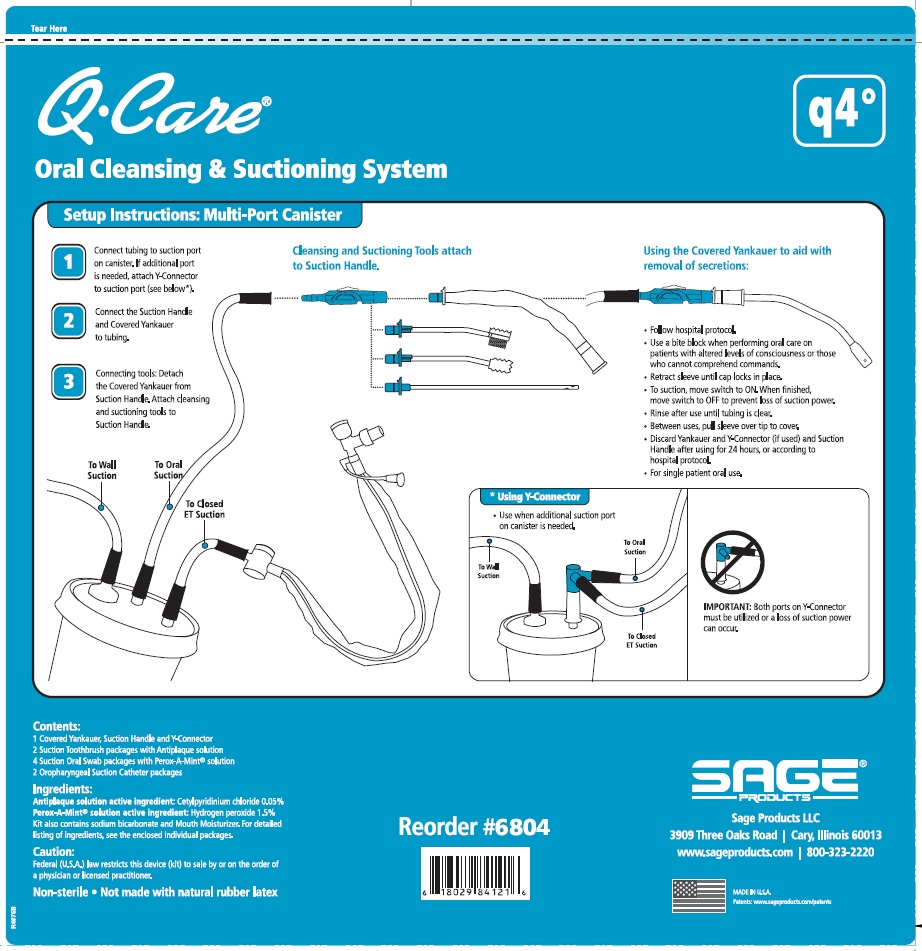
Label: QCARE ORAL CLEANSING AND SUCTIONING SYSTEM, Q4- cetylpyridinium chloride and hydrogen peroxide kit
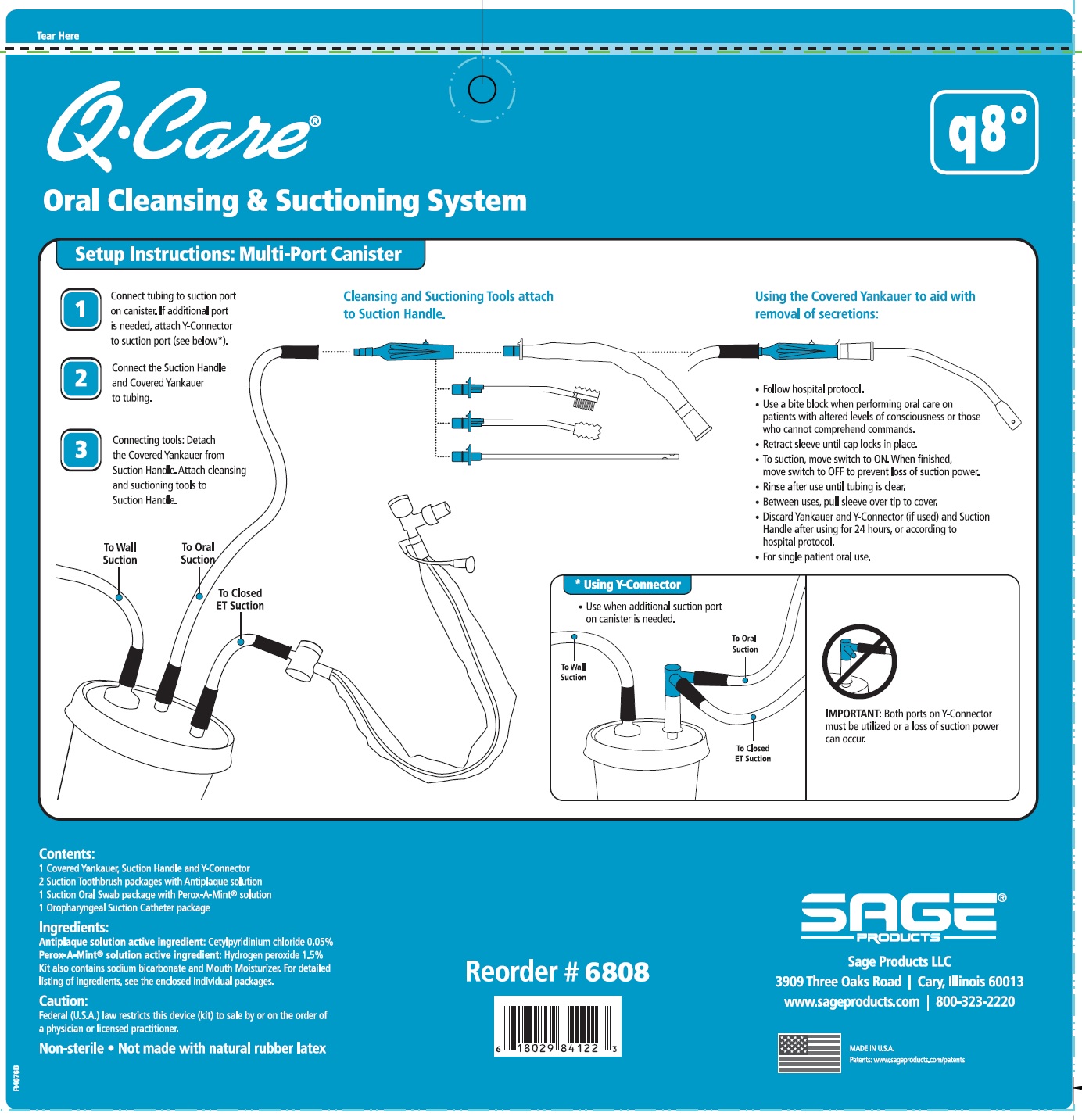
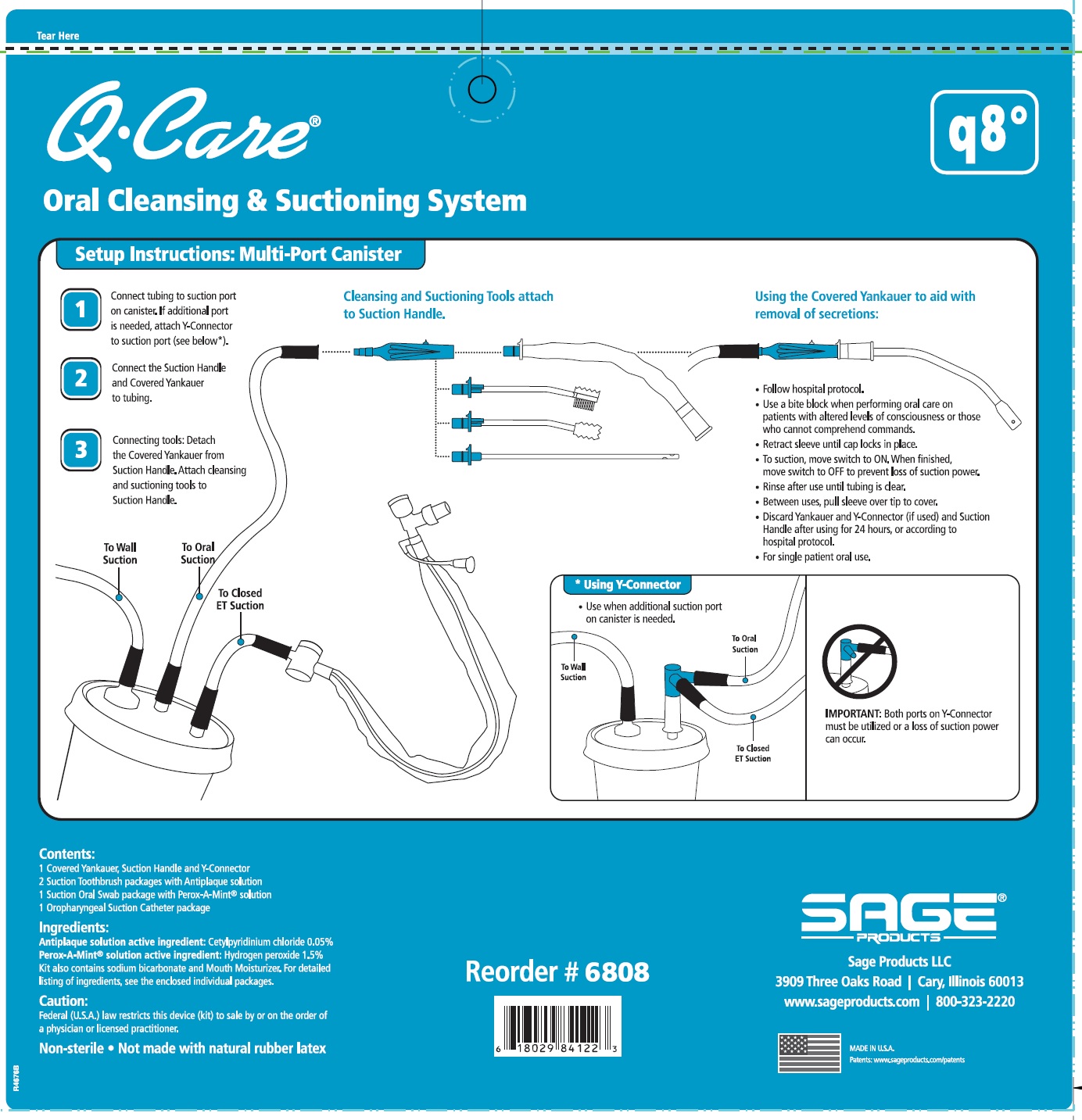
QCARE ORAL CLEANSING AND SUCTIONING SYSTEM, Q8- cetylpyridinium chloride and hydrogen peroxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 53462-075-60, 53462-275-60, 53462-804-60, 53462-808-60 - Packager: Sage Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- USES
- INDICATIONS & USAGE
-
WARNINGS
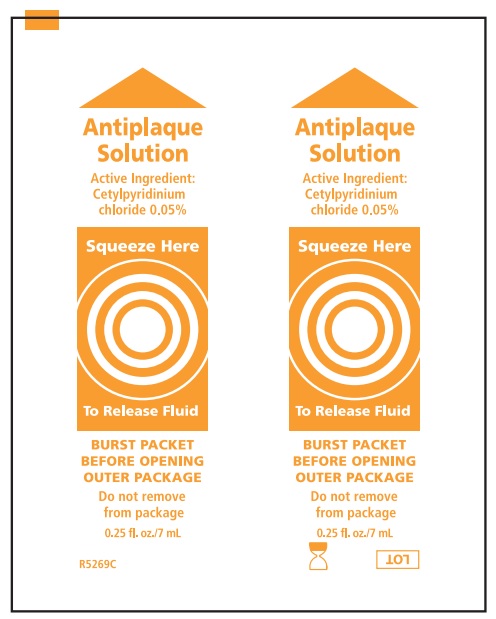
Suction Swab with Perox-A-Mint Solution:
Stop use and ask a doctor if:
- Sore mouth symptoms do not improve in 7 days.
- Swelling, rash or fever develops.
- Irritation, pain or redness persists or worsens.
Suction Toothbrush with Antiplaque Solution:
Stop use and ask a dentist if:
- Gingivitis, bleeding or redness persists for more than 2 weeks.
- You have painful or swollen gums, pus from the gum line, loose teeth or increasing spacing between the teeth. These may be signs or symptoms of periodontitis, a serious form of gum disease.
-
KEEP OUT OF REACH OF CHILDREN
Suction Swab with Perox-A-Mint Solution:
Keep out of reach of children.
If more than used for debriding is accidentally swallowed, get medical help or contact a Poison Control Center right away.Suction Toothbrush with Antiplaque Solution:
Keep out of reach of children under 6 years of age
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. - DOSAGE & ADMINISTRATION
-
DIRECTIONS
Suction Swab with Perox-A-Mint Solution:
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Suction Swab to Suction Handle.
- Clean teeth and oral cavity for approximately one minute.
- To suction, slide switch to ON. When finished, return switch to OFF.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Swab. Reattach Covered Yankauer to Suction Handle.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age: supervise use.
- Children under 3 years of age: consult a dentist or doctor.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Suction Toothbrush with Antiplaque Solution:
- Before opening, turn package over, burst solution packet with thumbs.
- Peel lid to open.
- Remove Mouth Moisturizer and Applicator Swab.
- Attach Toothbrush to Suction Handle.
- Clean teeth and oral cavity for approximately one minute.
- To suction, slide switch to ON. When finished, return switch to OFF.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Toothbrush. Reattach Covered Yankauer to Suction Handle.
- Place Mouth Moisturizer on Applicator Swab.
- Apply as needed to lips and inside mouth.
- Adults and children 12 years of age and older: use two times daily or as directed by a dentist. Do not swallow the solution.
- Children ages 6 to 12 years: supervise use.
- Children under 6 years of age: do not use.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
- Ensure foam is intact after use. If not, remove any particles from oral cavity.
Oropharyngeal Suction Catheter:
- Peel lid to open.
- Attach Suction Cather to Suction Handle.
- Suction secretions from the oropharyngeal cavity.
- To suction, slide switch to ON. When finished, return switch to OFF.
- To clear tubing, rinse with sterile saline or appropriate solution.
- Discard Suction Catheter. Reattach Covered Yankauer to Suction Handle.
- Use a bite block when performing oral care on patients with altered levels of consciousness or those who cannot comprehend commands.
Caution
- Federal (U.S.A.) law restricts this device to sale by or on the order of a physician or licensed practicioner.
-
Inactive Ingredients
Suction Swab with Perox-A-Mint Solution:
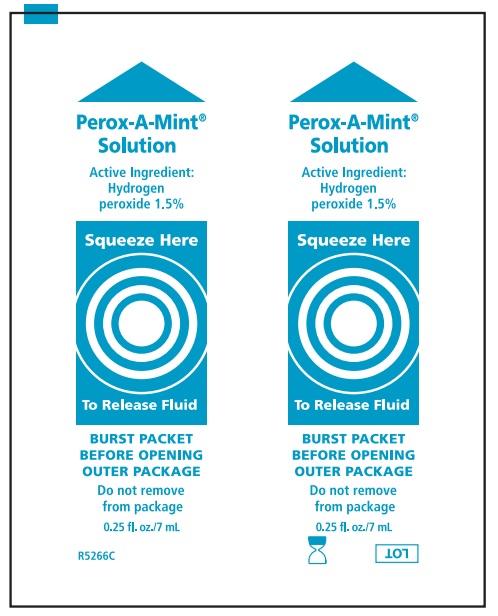
Water, menthol flavor, polysorbate 80, phosphoric acid, sodium saccharin, Blue 1 (CI 42090), Yellow 6 (CI 15985)
Suction Toothbrush with Antiplaque Solution:
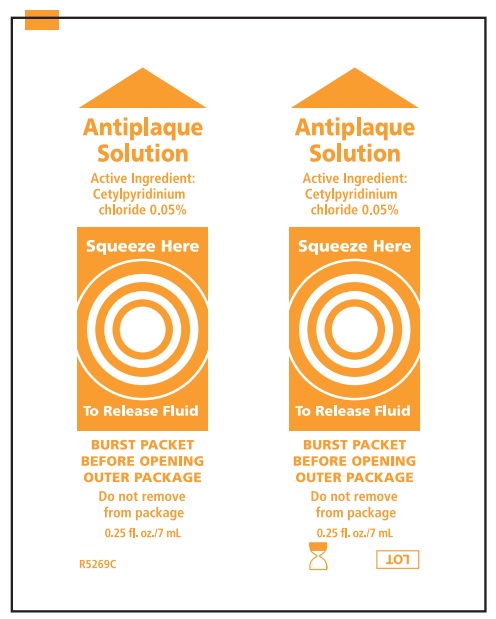
Water, sorbitol, peppermint flavor, potassium sorbate, polysorbate 80, polysorbate 20, citric acid, Blue 1 (CI42090)
- Questions?
- QCare Oral Cleansing and Suctioning System, Q4
- QCare Oral Cleansing and Suctioning System, Q8
- Antiplaque Solution, Perox-A-Mint
-
INGREDIENTS AND APPEARANCE
QCARE ORAL CLEANSING AND SUCTIONING SYSTEM, Q4
cetylpyridinium chloride and hydrogen peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-804 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-804-60 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 06/30/2004 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 4 PACKET 28 mL Part 2 2 PACKET 14 mL Part 3 1 Part 4 6 PACKET 12 g Part 1 of 4 PEROX-A-MINT SOLUTION
hydrogen peroxide mouthwashProduct Information Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 KIT 1 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Part 2 of 4 ANTIPLAQUE SOLUTION
cetylpyridinium chloride mouthwashProduct Information Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYSORBATE 20 (UNII: 7T1F30V5YH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Part 3 of 4 SODIUM BICARBONATE
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR SODIUM LAURYL SULFATE (UNII: 368GB5141J) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR WATER (UNII: 059QF0KO0R) INGR SACCHARIN SODIUM (UNII: SB8ZUX40TY) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 4 of 4 MOUTH MOISTURIZER
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR XYLITOL (UNII: VCQ006KQ1E) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR WATER (UNII: 059QF0KO0R) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6 in 1 KIT 1 2 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/30/2004 QCARE ORAL CLEANSING AND SUCTIONING SYSTEM, Q8
cetylpyridinium chloride and hydrogen peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-808 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-808-60 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 06/30/2004 04/09/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 7 mL Part 2 2 PACKET 14 mL Part 3 1 Part 4 3 PACKET 6 g Part 1 of 4 PEROX-A-MINT SOLUTION
hydrogen peroxide mouthwashProduct Information Item Code (Source) NDC:53462-075 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROGEN PEROXIDE (UNII: BBX060AN9V) (HYDROGEN PEROXIDE - UNII:BBX060AN9V) HYDROGEN PEROXIDE 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC:53462-075-60 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Part 2 of 4 ANTIPLAQUE SOLUTION
cetylpyridinium chloride mouthwashProduct Information Item Code (Source) NDC:53462-275 Route of Administration BUCCAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYSORBATE 20 (UNII: 7T1F30V5YH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC:53462-275-60 7 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 05/21/1998 Part 3 of 4 SODIUM BICARBONATE
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR SODIUM LAURYL SULFATE (UNII: 368GB5141J) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR WATER (UNII: 059QF0KO0R) INGR SACCHARIN SODIUM (UNII: SB8ZUX40TY) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Part 4 of 4 MOUTH MOISTURIZER
other oral hygiene productsProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR WATER (UNII: 059QF0KO0R) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) INGR CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) INGR COCONUT OIL (UNII: Q9L0O73W7L) INGR XYLITOL (UNII: VCQ006KQ1E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 in 1 KIT 1 2 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 06/30/2004 04/17/2018 Labeler - Sage Products LLC (054326178) Registrant - Sage Products LLC (054326178)