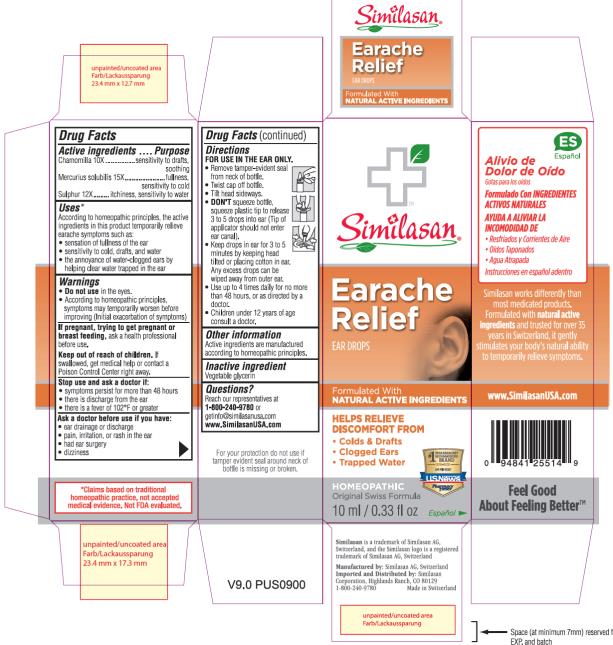
Label: EARACHE RELIEF- chamomilla and mercurius solubilis and sulphur solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 59262-277-11 - Packager: Similasan Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 8, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Active ingredients
- Purpose
- Active ingredients
- Purpose
- Uses*
-
Warnings
• Do not use in the eyes.
• According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms)
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
-
Directions
FOR USE IN THE EAR ONLY.
• Remove tamper-evident seal from neck of bottle.
• Twist cap off bottle.
• Tilt head sideways.
• DON'T squeeze bottle, squeeze plastic tip to release 3 to 5 drops into ear (Tip of applicator should not enter ear canal).
• Keep drops in ear for 3 to 5 minutes by keeping head tilted or placing cotton in ear. Any excess drops can be wiped away from outer ear.
• Use up to 4 times daily for no more than 48 hours, or as directed by a doctor.
• Children under 12 years of age consult a doctor.
- Other information
- Inactive ingredient
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EARACHE RELIEF
chamomilla and mercurius solubilis and sulphur solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-277 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 10 [hp_X] in 10 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 15 [hp_X] in 10 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 10 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-277-11 10 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 07/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 07/02/2013 Labeler - Similasan Corporation (111566530) Registrant - Similasan AG (481545754)