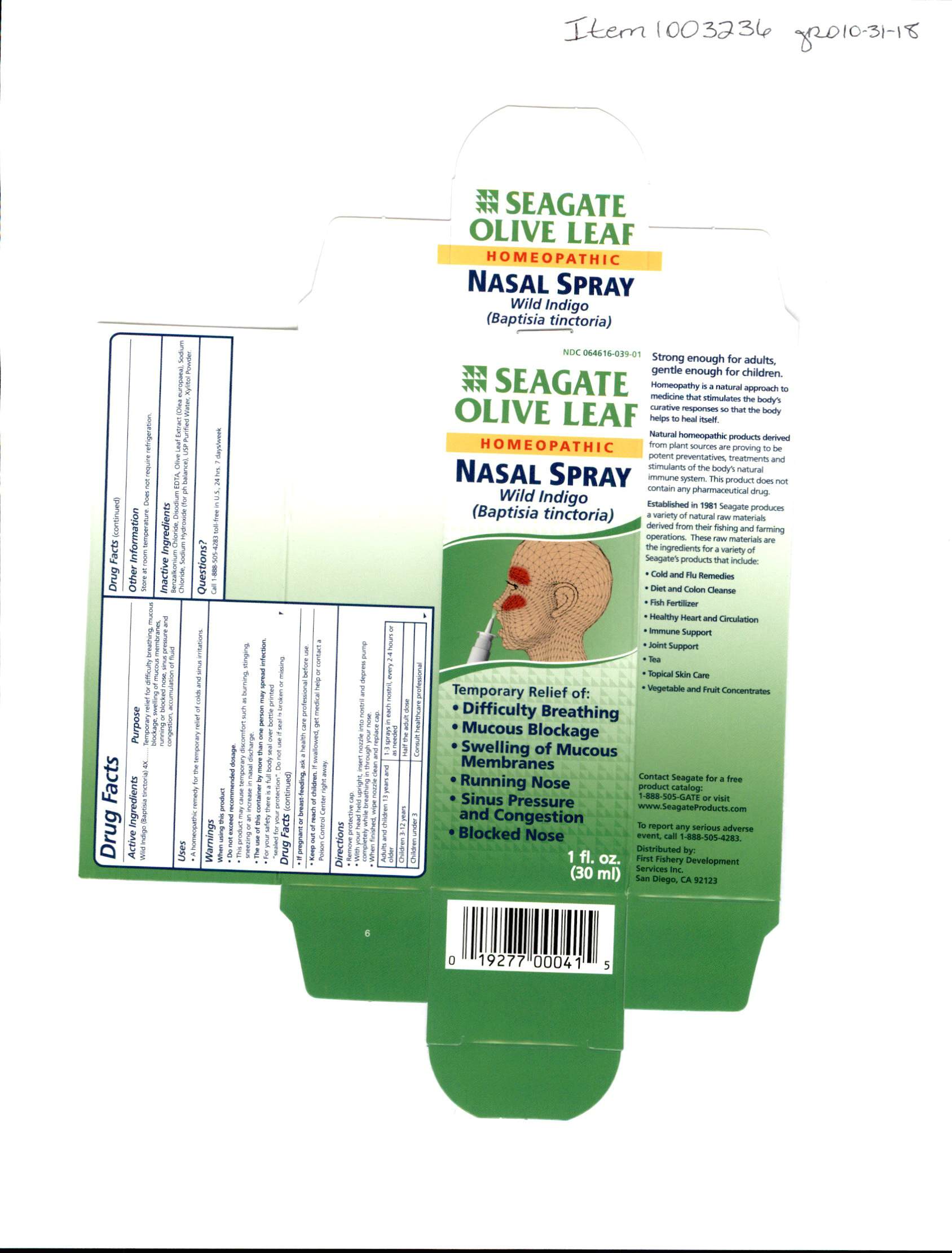
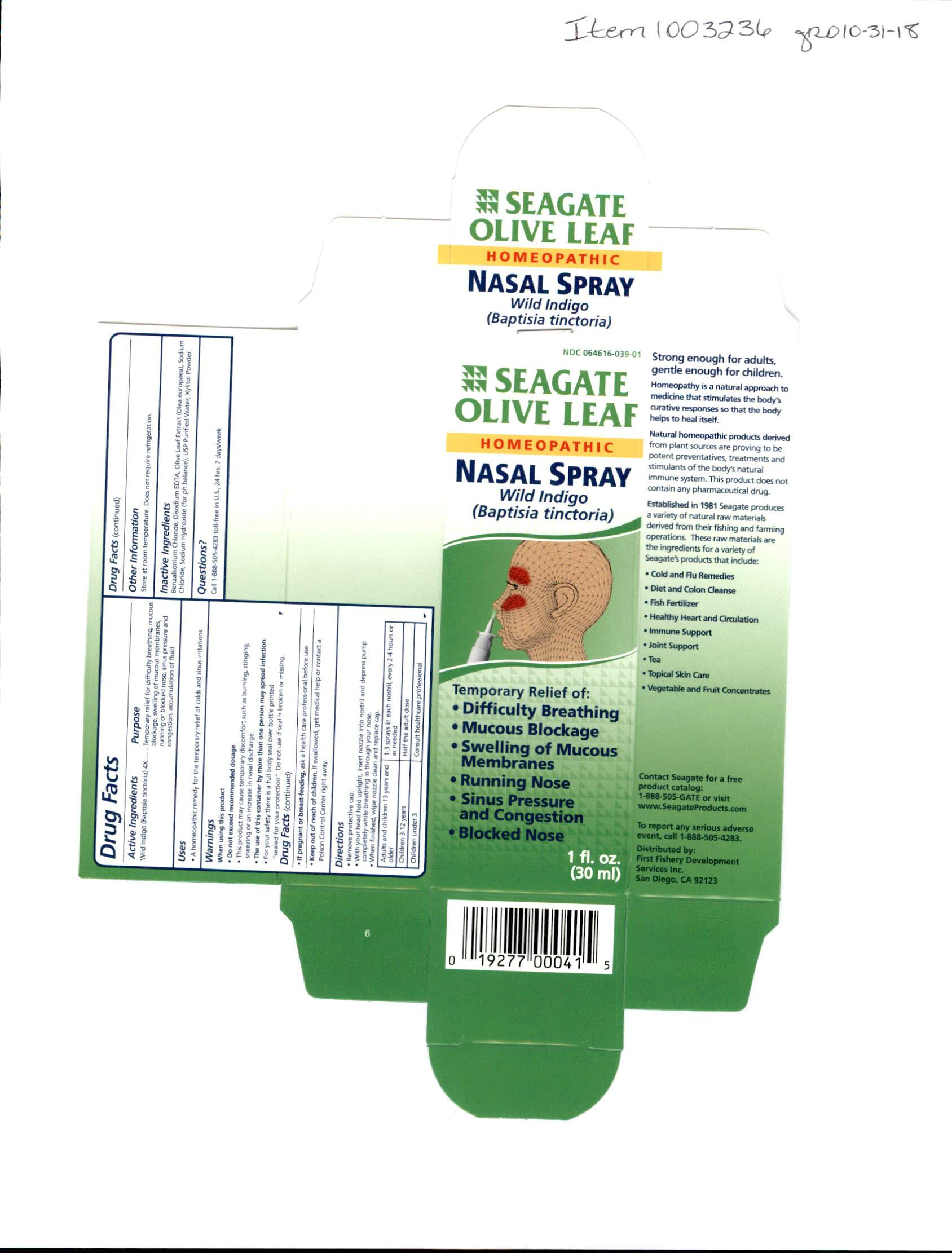
Label: OLIVE LEAF NASAL- nasal spray spray
- NDC Code(s): 64616-039-01, 64616-039-02
- Packager: Vitality Works, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Olive Leaf Nasal Spray
- Olive Leaf Nasal Spray
-
Olive Leaf Nasal Spray
Do not exceed recommended dosage. This product may cause temporary discomfort such as burning, stinging, sneezing or an increase in nasal doscharge. The use of this container by more than one person my spread infection. For your safety there is a full body seal over bottle printed "sealed for your protection". Do not use if seal is broken or missing. If pregnant or breastfeeding ask a health care professional before use.
- Olive Leaf Nasal Spray
- Olive Leaf Nasal Spray
-
Olive Leaf Nasal Spray
Remove the protective cap. With your head held upright insert nozzel into nostril and depress pump completely while breathing in through your nose. When finished, wipe nozzel clean and replace cap.
Adults and children 13 years and older, use 1-3 sprays in each nostril every 2-4 hours or as needed. Children 3-12 years, half the adult dosage. children under 3 years of age, consult a health care professional.
- Olive Leaf Nasal Spray
- QUESTIONS
- Olive Leaf Nasal Spray
-
INGREDIENTS AND APPEARANCE
OLIVE LEAF NASAL
nasal spray sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64616-039 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BAPTISIA TINCTORIA (UNII: 5K1UO2888Y) (BAPTISIA TINCTORIA - UNII:5K1UO2888Y) BAPTISIA TINCTORIA 4 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) XYLITOL (UNII: VCQ006KQ1E) SODIUM HYDROXIDE (UNII: 55X04QC32I) COBALT DISODIUM EDETATE (UNII: 3EY1Y2QRLI) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64616-039-02 1 in 1 CARTON 01/07/2003 1 NDC:64616-039-01 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/07/2003 Labeler - Vitality Works, Inc. (137752817) Registrant - Vitality Works, Inc. (137752817) Establishment Name Address ID/FEI Business Operations Vitality Works, Inc. 137752817 manufacture(64616-039)