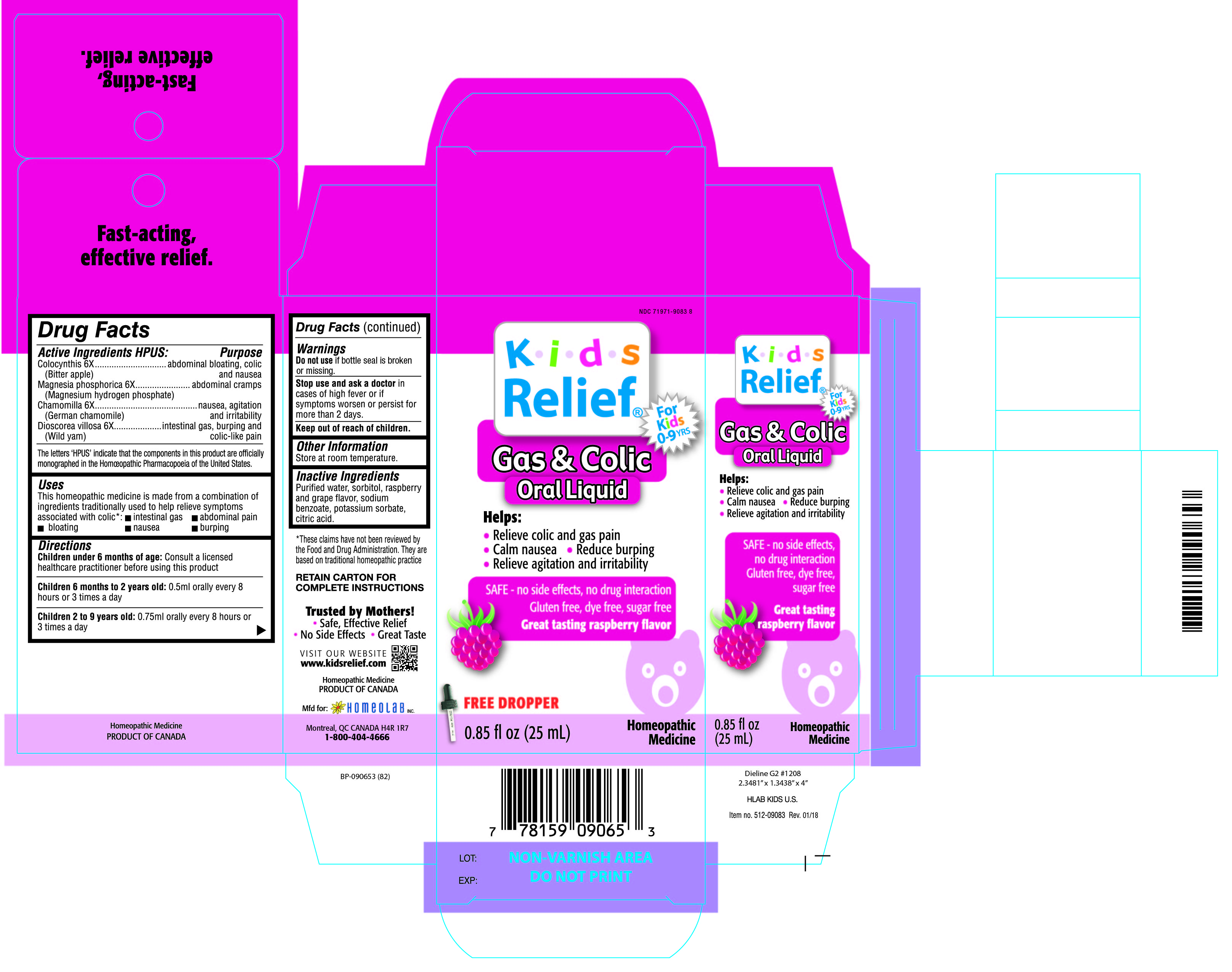
Label: KIDS RELIEF- colocynthis, magnesia phosphorica, chamomilla, dioscorea villosa liquid
- NDC Code(s): 71971-9083-8
- Packager: Homeolab International (Canada) inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 16, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients HPUS:
Colocynthis 6X
(Bitter apple)
Magnesia phosphorica 6X
(Magnesium hydrogen phosphate)
Chamomilla 6X
(German chamomille)
Dioscorea villosa 6X
(Wild yam)
The letters 'HPUS' indicate that the component in this product is officially
monographed in the Homeopathic Pharmacopoeia of the United States)
-
PURPOSE
Purpose
Colocynthis 6X...........................................abdominal bloating, colic and nausea
(Bitter apple)
Magnesia phosphorica 6X ----------------------abdominal cramps
(Magnesium hydrogen phosphate)
Chamomilla 6X ..........................................nausea, agitation and irritability
(German chamomille)
Dioscorea villosa 6X ...................................intestinal gas, burping and colic-like pain
(Wild yam)
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Kids Relief Gas & Colic Oral Liquid
- Kids Relief Gas & Colic Oral Liquid
-
INGREDIENTS AND APPEARANCE
KIDS RELIEF
colocynthis, magnesia phosphorica, chamomilla, dioscorea villosa liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71971-9083 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 6 [hp_X] in 25 mL MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 6 [hp_X] in 25 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 6 [hp_X] in 25 mL DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) (DIOSCOREA VILLOSA ROOT - UNII:IWY3IWX2G8) DIOSCOREA VILLOSA TUBER 6 [hp_X] in 25 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID ACETATE (UNII: DSO12WL7AU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71971-9083-8 1 in 1 CARTON 03/03/2017 1 25 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/03/2017 Labeler - Homeolab International (Canada) inc (203639455)