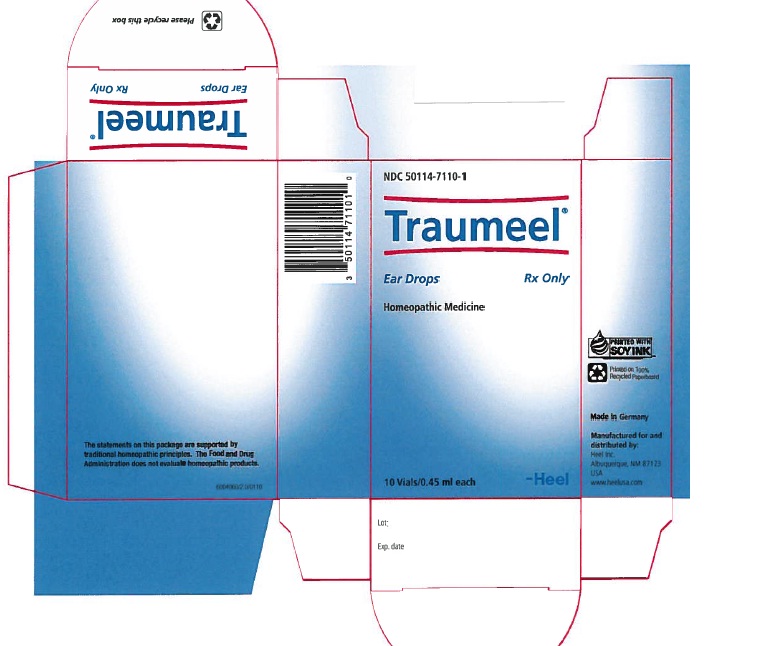
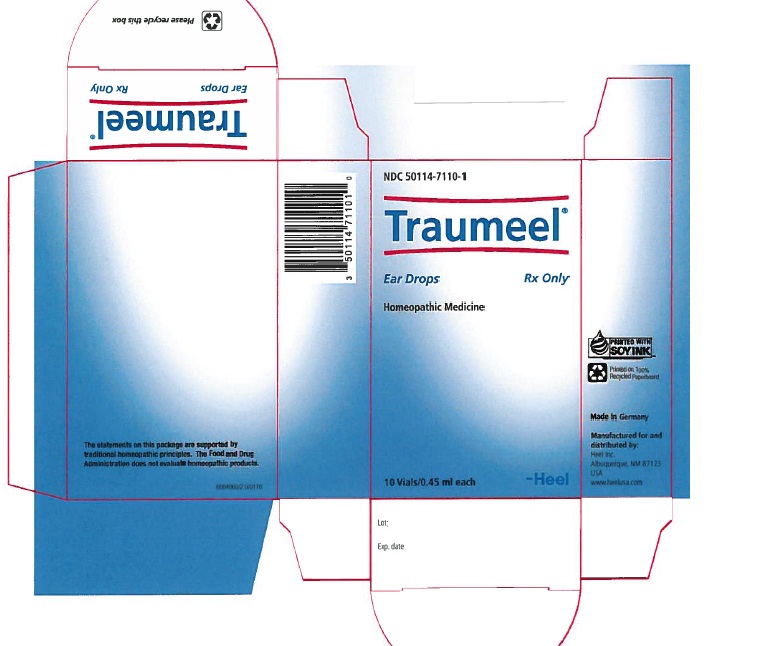
Label: TRAUMEEL- arnica montana root and atropa belladonna and aconitum napellus and hypericum perforatum and calendula officinalis flowering top and matricaria recutita and achillea millefolium and calcium sulfide and comfrey root and bellis perennis and mercurius solubilis and echinacea, unspecified and echinacea purpurea and hamamelis virginiana root bark/stem bark solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 50114-7110-1 - Packager: Heel Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 23, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Traumeel Ear Drops Rx is a liquid homeopathic drug for otic administration. Each 0.45 ml vial contains Active Ingredients: Arnica montana, radix 3X 9.9 mcl, Belladonna 3X 9.9 mcl, Aconitum napellus 3X 9.54 mcl, Hypericum perforatum 3X 2.97 mcl, Calendula officinalis 2X 0.99 mcl, Chamomilla 3X 0.99 mcl, Millefolium 3X 0.99 mcl, Hepar sulphuris calcareum 6X 0.99 mcl, Symphytum officinale 6X 0.99 mcl, Bellis perennis 2X 0.495 mcl, Mercurius solubilis 6X 0.495 mcl, Echinacea 2X 0.247 mcl, Echinacea purpurea 2X 0.247 mcl, Hamamellis virginiana 1X 0.099 mcl Inactive Ingredients: Isotonic Sodium Chloride Solution.
- INDICATIONS AND USAGE
- DOSAGE AND ADMINISTRATION
-
WARNINGS AND PRECAUTIONS
No harmful or potentially harmful side effects are known. Traumeel ear drops is generally well-tolerated, however if symptoms persist or worsen, discontinue use.
To report SUSPECTED ADVERSE REACTIONS, contact Heel Inc. at 1.800.920.9203 or info@heelusa.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRAUMEEL
arnica montana root and atropa belladonna and aconitum napellus and hypericum perforatum and calendula officinalis flowering top and matricaria recutita and achillea millefolium and calcium sulfide and comfrey root and bellis perennis and mercurius solubilis and echinacea, unspecified and echinacea purpurea and hamamelis virginiana root bark/stem bark solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:50114-7110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 3 [hp_X] in 0.45 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 3 [hp_X] in 0.45 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 3 [hp_X] in 0.45 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 3 [hp_X] in 0.45 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 2 [hp_X] in 0.45 mL MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 3 [hp_X] in 0.45 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 3 [hp_X] in 0.45 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM CATION - UNII:2M83C4R6ZB, SULFIDE ION - UNII:G15I91XETI) CALCIUM SULFIDE 6 [hp_X] in 0.45 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 0.45 mL BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 2 [hp_X] in 0.45 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 6 [hp_X] in 0.45 mL ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) (ECHINACEA, UNSPECIFIED - UNII:4N9P6CC1DX) ECHINACEA, UNSPECIFIED 2 [hp_X] in 0.45 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 2 [hp_X] in 0.45 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 1 [hp_X] in 0.45 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50114-7110-1 10 in 1 CARTON 1 0.45 mL in 1 VIAL, SINGLE-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/30/2002 Labeler - Heel Inc (102783016) Establishment Name Address ID/FEI Business Operations Holopack-Verpackungstechnik-GmbH 313222457 manufacture