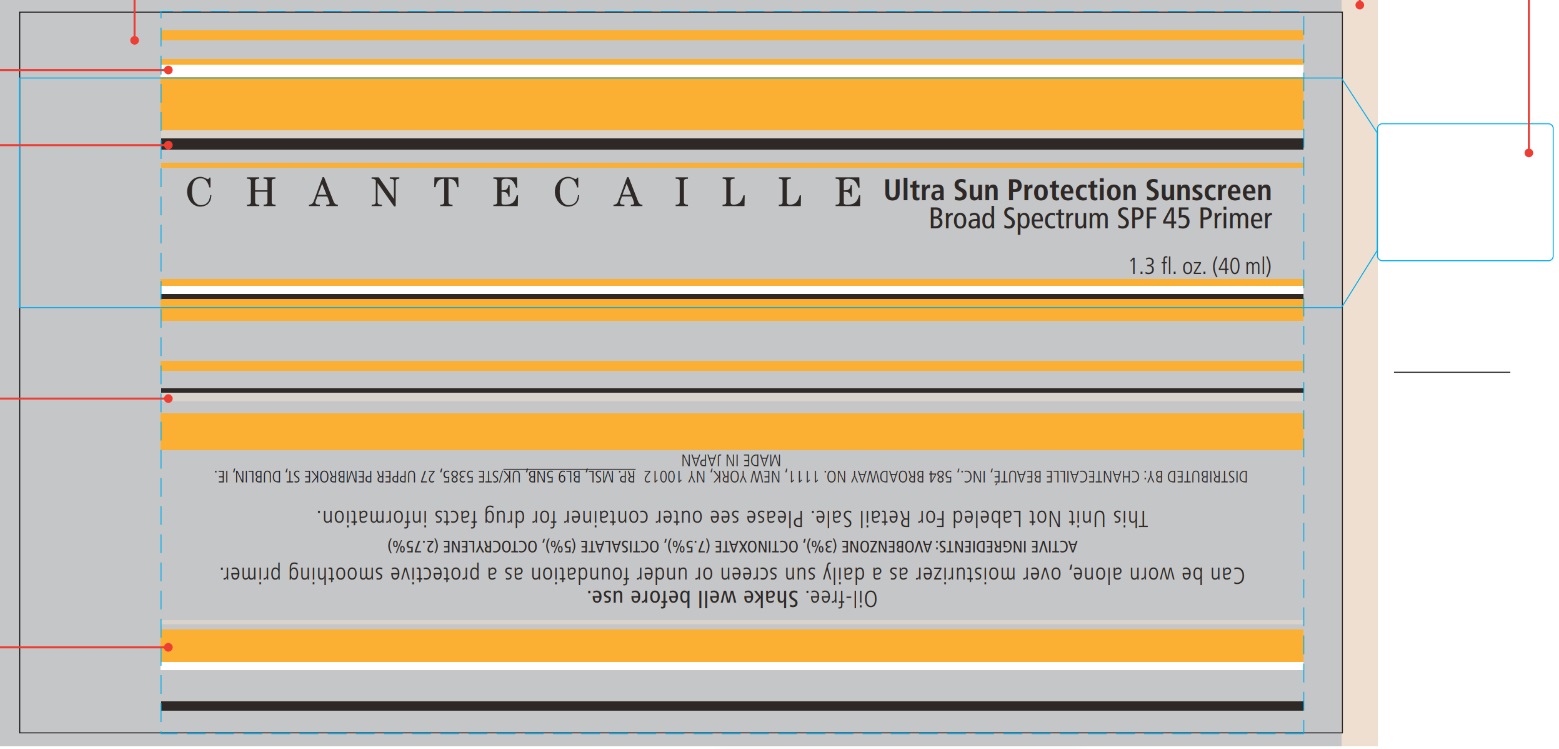
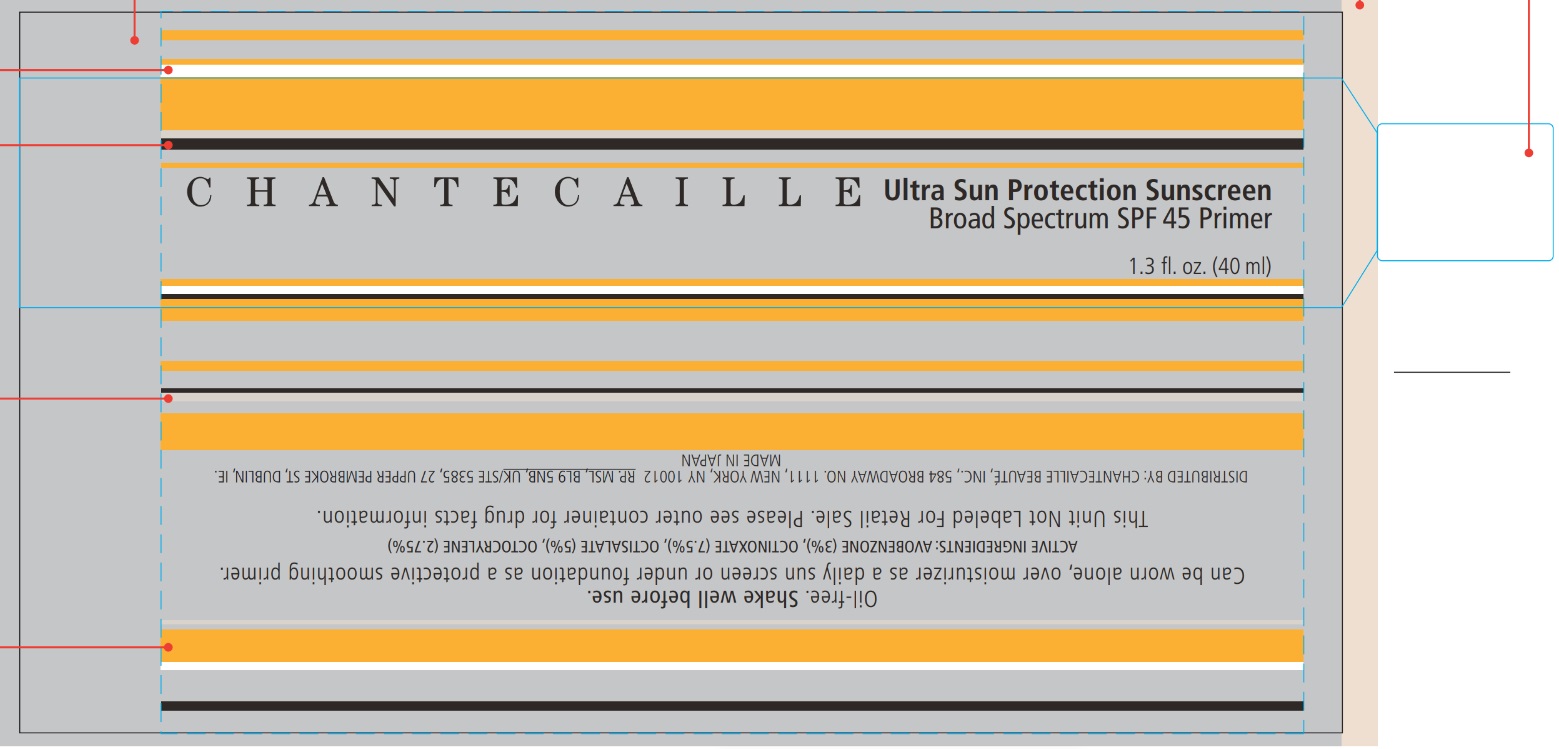
Label: CHANTECAILLE ULTRA SUN PROTECTION SUNSCREEN BROAD SPECTRUM SPF 45- avobenzone, octinoxate, octisalate, octocrylene lotion
- NDC Code(s): 42893-013-00
- Packager: Chantecaille Beaute Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- CHANTECAILLE Ultra Sun Protection Sunscreen Broad Spectrum SPF 45
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- Warnings
- Keep Out Of Reach Of Children.
-
DIRECTIONS
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months of age: Ask a doctor
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
-
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- OTHER INFORMATION
-
INACTIVE INGREDIENTS:
DAMASK ROSE (ROSA DAMASCENA) FLOWER WATER, C12-15 ALKYL BENZOATE, BUTYLENE GLYCOL, POLYMETHYLSILSESQUIOXANE, POLYMETHYL METHACRYLATE, ISONONYL ISONONANOATE, METHYL METHACRYLATE CROSSPOLYMER, GLYCERIN, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, DICAPRYLYL CARBONATE, PENTYLENE GLYCOL, POLYGLYCERYL-6 POLYRICINOLEATE, PHENOXYETHANOL, SODIUM CHLORIDE, POLYGLYCERYL-2 ISOSTEARATE, SORBITAN SESQUIISOSTEARATE, TETRASODIUM EDTA, TOCOPHEROL, LEMON BALM (MELISSA OFFICINALIS) LEAF EXTRACT, STEARYL GLYCYRRHETINATE, CARNOSINE, LECITHIN, TEA (CAMELLIA SINENSIS) LEAF EXTRACT, YOSHINO CHERRY (PRUNUS YEDOENSIS) LEAF EXTRACT, CYCLODEXTRIN
- Package labeling
-
INGREDIENTS AND APPEARANCE
CHANTECAILLE ULTRA SUN PROTECTION SUNSCREEN BROAD SPECTRUM SPF 45
avobenzone, octinoxate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42893-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 27.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ROSA DAMASCENA FLOWER OIL (UNII: 18920M3T13) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) GLYCERIN (UNII: PDC6A3C0OX) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) PENTYLENE GLYCOL (UNII: 50C1307PZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYGLYCERYL-2 MONOISOSTEARATE (UNII: 7B8OE71MQC) EDETATE SODIUM (UNII: MP1J8420LU) TOCOPHEROL (UNII: R0ZB2556P8) OCIMUM AFRICANUM LEAF (UNII: 0231102WJO) STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) CARNOSINE (UNII: 8HO6PVN24W) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) TEA LEAF (UNII: GH42T47V24) PRUNUS X YEDOENSIS LEAF (UNII: 1Z125GA907) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42893-013-00 40 mL in 1 TUBE; Type 0: Not a Combination Product 07/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/30/2014 Labeler - Chantecaille Beaute Inc (095270166)