Label: OXYGEN gas
- NDC Code(s): 52003-911-20
- Packager: Messer Canada Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Messer
Gases for Life
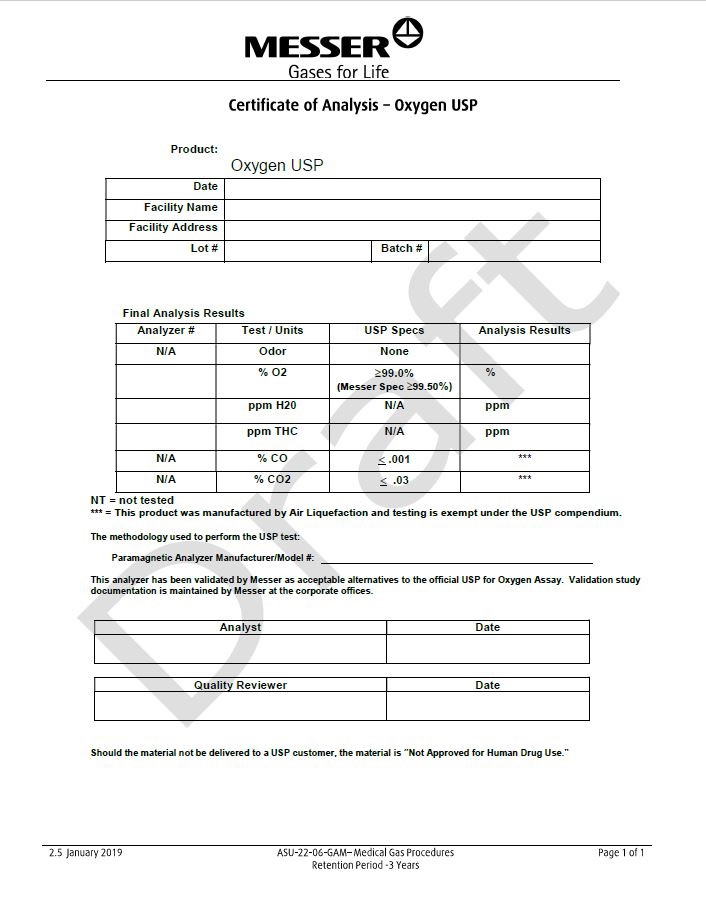
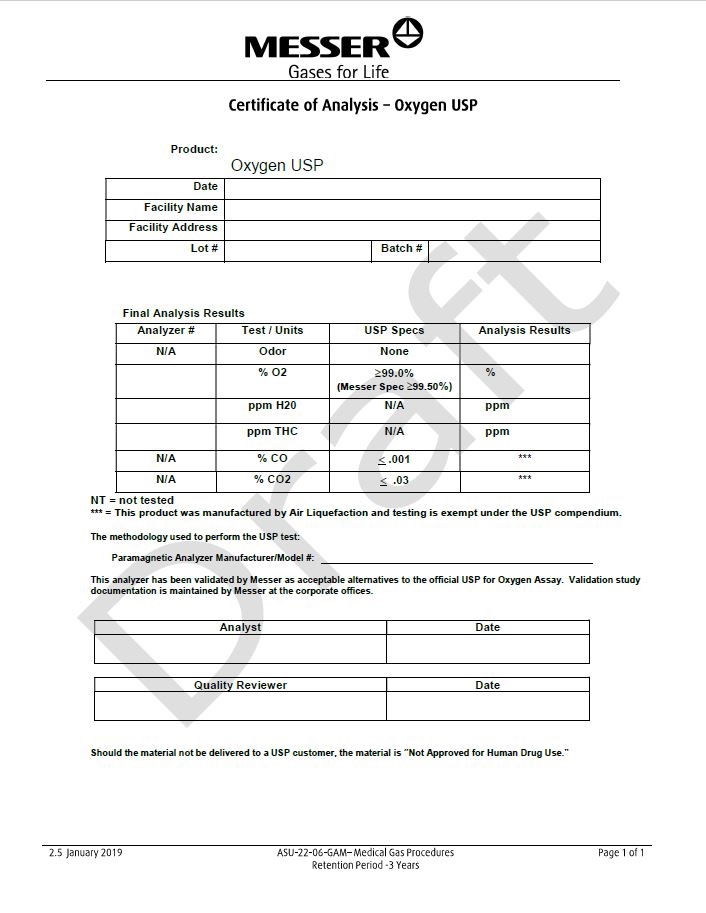
Certicate of Analysis - Oxygen USP
Product: Oxygen USP
Date
Facility Name
Facility Address
Lot#
Batch #
Final Analysis Results
Analyzer #
Test/Units
USP Specs
Analysis Results
N/A
Odor
None
% O2
> 99.0%
(Messer Spec >99.50%)%
ppm H2O
N/A
ppm
ppm THC
N/A
ppm
N/A
% CO
≤ .001
***
N/A
% CO2
≤ .03
***
NT= not tested
*** = This product was manufactured by Air Liquefaction and testing is exempt under the USP compendium.
The methodology used to perform the USP test:
Paramagnetic Analyzer Manufacturer / Model# : _______________________________________________This analyzer has been validated by Messer as acceptable alternatives to the official USP for Oxygen Assay. Validation study documentation is maintained by Messer at the corporate offices.
Analyst
Date
Quality Reviewer
Date
Should the material not be delivered to a USP customer, the material is “Not Approved for Human Drug Use.”
2.5 January 2019
ASU-22-06-GAM-Medical Gas Procedures
Page 1 of 1
Retention Period – 3 Years
-
INGREDIENTS AND APPEARANCE
OXYGEN
oxygen gasProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:52003-911 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Oxygen (UNII: S88TT14065) (Oxygen - UNII:S88TT14065) Oxygen 995 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52003-911-20 18144 L in 1 TANK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141373 01/01/1960 Labeler - Messer Canada Inc. (201613189) Registrant - Messer Canada Inc. (201613189) Establishment Name Address ID/FEI Business Operations Messer Canada Inc. 244231937 manufacture, api manufacture