Label: CERAMIDE PREMIER INTENSE MOISTURE AND RENEWAL ACTIVATION BROAD SPECTRUM SPF 30- octinoxate, octisalate, oxybenzone, octocrylene, and avobenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 67938-1145-1, 67938-1145-2 - Packager: Elizabeth Arden, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 30, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
This rich, replenishing moisture cream with skin identical Ceramide Triple Complex hydrates deeply to restore skin's natural protective barrier and ease the look of fine lines and wrinkles. Nutrient rich sea minerals including calcium help support skin’s natural processes to fortify thin, fragile looking skin. Our patented retinyl complex is clinically proven to optimize surface cell turnover to improve the appearance of age spots, discolorations and overall radiance.
-
DOSAGE AND ADMINISTRATION
To Use: Apply to cleansed face and throat.
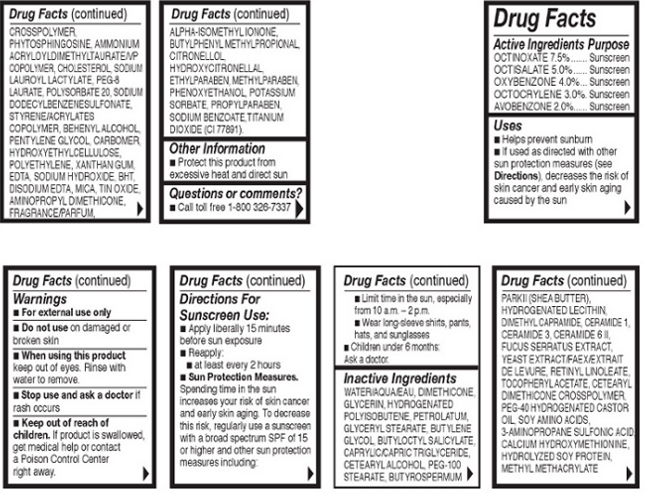
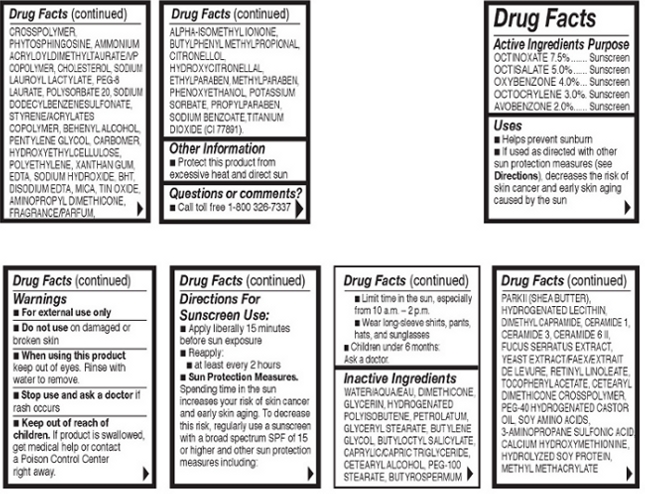
Directions For Sunscreen Use:Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
Sun Protection Measures:Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease mthis risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. – 2 p.m. Wear long-sleeve shirts, pants, hats, and sunglasses
Children under 6 months: Ask a doctor.
- OTC - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients: Water/Aqua/Eau, Dimethicone, Glycerin, Hydrogenated Polyisobutene, Petrolatum, Glyceryl Stearate, Butylene Glycol, Butyloctly Salicylate, Caprylic/Capric Triglyceride, Cetearyl Alochol, PEG-100 Stearate, Butyrospermum Parkii (Shea Butter), Hydrogenated Lecithin, Dimethylcapramide, Ceramide 1, Ceramide 3, Ceramide 6 II, Fucus Serratus Extract, Yeast Extract/Faex/Extrait de Levure, Retinyl Lonoleate, Tocopheryl Acetate, Cetearyl Dimethicone Crosspolymer, PEG-40 Hydrogenated Castor Oil, Soy Amino Acids, 3-Aminopropane Sulfonic Acid, Calcium Hydroxymethionine, Hydrolyzed Soy Protein, Methyl Methacrylate Crosspolymer, Phytosphingosine, Ammonium Acryloyldimethyltaurate/VP Copolymer, Cholesterol, Sodium Lauroyl Lactylate, PEG-8 Laurate, Polysorbate 20, Sodium Dodecylbenzenesulfonate, Styrene/Acrylates Copolymer, Behenyl Alcohol, Pentylene Glycol, Carbomer, Hydroxyethylcellulose, Polyethylene, Xanthan Gum, EDTA, Sodium Hydroxide, BHT, Disodium EDTA, Mica, Tin Oxide, Aminopropyl Dimethicone, Fragrance/Parfum, Alpha-Isomethyl Ionone, Butylpheyl Methylpropional, Citronellol, Hydroxycitronellal, Ethylparaben, Methylparaben, Phenoxyethanol, Potassium Sorbate, Propylparaben, Sodium Benzoate, Titanium Dioxide (CI 77891).
- INDICATIONS AND USAGE
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - WHEN USING
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAMIDE PREMIER INTENSE MOISTURE AND RENEWAL ACTIVATION BROAD SPECTRUM SPF 30
octinoxate, octisalate, oxybenzone, octocrylene, and avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67938-1145 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.68 g in 49 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 2.45 g in 49 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1.96 g in 49 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.47 g in 49 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.98 g in 49 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) PETROLATUM (UNII: 4T6H12BN9U) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-100 STEARATE (UNII: YD01N1999R) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) DIMETHYL CAPRAMIDE (UNII: O29Y6X2JEZ) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE 3 (UNII: 4370DF050B) CERAMIDE 6 II (UNII: F1X8L2B00J) FUCUS SERRATUS (UNII: V8K40WNW5B) YEAST (UNII: 3NY3SM6B8U) RETINYL LINOLEATE (UNII: 61911N8D6W) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) TRAMIPROSATE (UNII: 5K8EAX0G53) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CHOLESTEROL (UNII: 97C5T2UQ7J) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) PEG-8 LAURATE (UNII: 762O8IWA10) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) DOCOSANOL (UNII: 9G1OE216XY) PENTYLENE GLYCOL (UNII: 50C1307PZG) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) XANTHAN GUM (UNII: TTV12P4NEE) EDETIC ACID (UNII: 9G34HU7RV0) SODIUM HYDROXIDE (UNII: 55X04QC32I) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) EDETATE DISODIUM (UNII: 7FLD91C86K) MICA (UNII: V8A1AW0880) STANNIC OXIDE (UNII: KM7N50LOS6) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) ETHYLPARABEN (UNII: 14255EXE39) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BENZOATE (UNII: OJ245FE5EU) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67938-1145-1 1 in 1 JAR 1 NDC:67938-1145-2 49 g in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/22/2011 Labeler - Elizabeth Arden, Inc (849222187)