Label: HLAL RDNA CONSTRUCT IN SBC LAL-C CHICKENS- hlal rdna construct in sbc lal-c chicken not applicable
- NDC Code(s): 25682-020-01
- Packager: Alexion Pharmaceuticals, Inc.
- Category: RECOMBINANT DEOXYRIBONUCLEIC ACID CONSTRUCT LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated June 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
1. PRODUCT IDENTIFICATION
hLAL rDNA construct integrated at a single site (the SYN LAL-C site in chromosome 6) as a single copy, in a specific, diploid line (SBC LAL-C1) of hemizygous and homozygous domestic chickens (Gallus gallus) derived from the lineage progenitor XLL 109, expressing a recombinant human lysosomal acid lipase (rhLAL) encoding gene such that rhLAL protein (intended for the treatment of humans) is present in their egg whites.
- 1
- SBC LAL-C is the name Alexion designated for their lineage of chickens, and is not representative of the regulated article, which is subject to FDA approval. The FDA-regulated article subject to this approval is the intentional genomic alteration (hLAL rDNA construct) in the chickens.
- 2. WARNINGS AND PRECAUTIONS
-
3. ANIMAL SAFETY
Data from the following comparisons of SBC LAL-C chickens to comparator chickens (without an intentional genomic alteration; IGA) demonstrated a lack of adverse effect due to the rDNA construct, or to the recombinant human LAL protein, on the SBC LAL-C chicken.
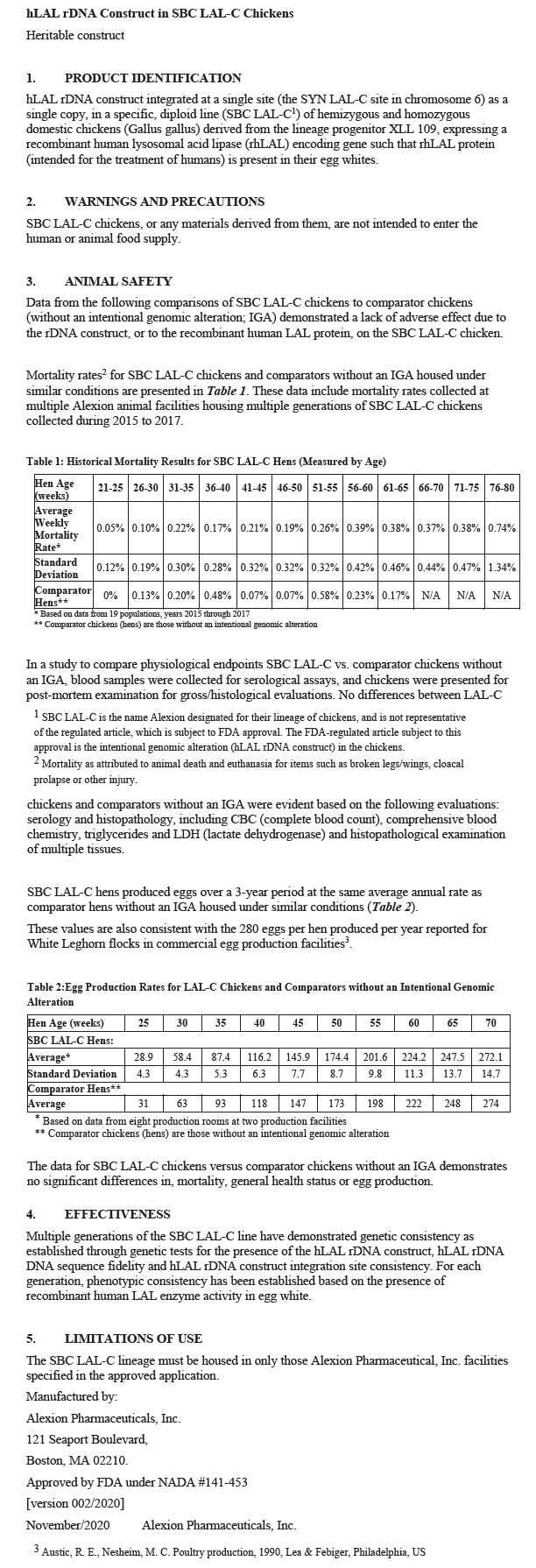
Mortality rates2 for SBC LAL-C chickens and comparators without an IGA housed under similar conditions are presented in Table 1. These data include mortality rates collected at multiple Alexion animal facilities housing multiple generations of SBC LAL-C chickens collected during 2015 to 2017.
Table 1: Historical Mortality Results for SBC LAL-C Hens (Measured by Age) Hen Age (weeks) 21-25 26-30 31-35 36-40 41-45 46-50 51-55 56-60 61-65 66-70 71-75 76-80 Average Weekly Mortality Rate* 0.05% 0.10% 0.22% 0.17% 0.21% 0.19% 0.26% 0.39% 0.38% 0.37% 0.38% 0.74% Standard Deviation 0.12% 0.19% 0.30% 0.28% 0.32% 0.32% 0.32% 0.42% 0.46% 0.44% 0.47% 1.34% Comparator Hens† 0% 0.13% 0.20% 0.48% 0.07% 0.07% 0.58% 0.23% 0.17% N/A N/A N/A In a study to compare physiological endpoints SBC LAL-C vs. comparator chickens without an IGA, blood samples were collected for serological assays, and chickens were presented for post-mortem examination for gross/histological evaluations. No differences between LAL-C chickens and comparators without an IGA were evident based on the following evaluations: serology and histopathology, including CBC (complete blood count), comprehensive blood chemistry, triglycerides and LDH (lactate dehydrogenase) and histopathological examination of multiple tissues.
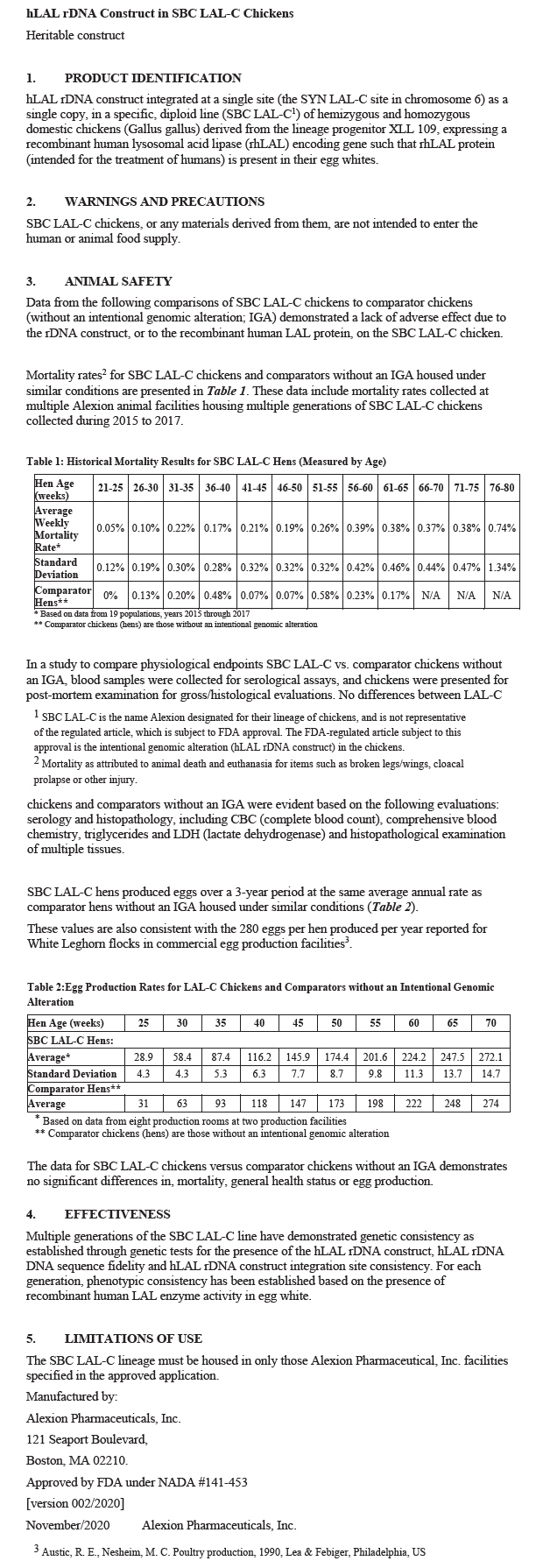
SBC LAL-C hens produced eggs over a 3-year period at the same average annual rate as comparator hens without an IGA housed under similar conditions (Table 2).
These values are also consistent with the 280 eggs per hen produced per year reported for White Leghorn flocks in commercial egg production facilities3.
Table 2:Egg Production Rates for LAL-C Chickens and Comparators without an Intentional Genomic Alteration Hen Age (weeks) 25 30 35 40 45 50 55 60 65 70 SBC LAL-C Hens: Average* 28.9 58.4 87.4 116.2 145.9 174.4 201.6 224.2 247.5 272.1 Standard Deviation 4.3 4.3 5.3 6.3 7.7 8.7 9.8 11.3 13.7 14.7 Comparator Hens† Average 31 63 93 118 147 173 198 222 248 274 The data for SBC LAL-C chickens versus comparator chickens without an IGA demonstrates no significant differences in, mortality, general health status or egg production.
-
4. EFFECTIVENESS
Multiple generations of the SBC LAL-C line have demonstrated genetic consistency as established through genetic tests for the presence of the hLAL rDNA construct, hLAL rDNA DNA sequence fidelity and hLAL rDNA construct integration site consistency. For each generation, phenotypic consistency has been established based on the presence of recombinant human LAL enzyme activity in egg white.
- 5. LIMITATIONS OF USE
- SPL UNCLASSIFIED SECTION
- hLAL rDNA Construct in SBC LAL-C Chickens
-
INGREDIENTS AND APPEARANCE
HLAL RDNA CONSTRUCT IN SBC LAL-C CHICKENS
hlal rdna construct in sbc lal-c chicken not applicableProduct Information Product Type RECOMBINANT DEOXYRIBONUCLEIC ACID CONSTRUCT LABEL Item Code (Source) NDC:25682-020 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HLAL RDNA CONSTRUCT IN SBC LAL-C CHICKEN (UNII: MG6AU4C2HB) (HLAL RDNA CONSTRUCT IN SBC LAL-C CHICKEN - UNII:MG6AU4C2HB) HLAL RDNA CONSTRUCT IN SBC LAL-C CHICKEN 1 [arb'U] in 1 [arb'U] Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:25682-020-01 1 [arb'U] in 1 NOT APPLICABLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141453 12/08/2015 Labeler - Alexion Pharmaceuticals, Inc. (789359510)