Label: TYVASO- treprostinil inhalant
- NDC Code(s): 66302-206-01, 66302-206-02, 66302-206-03, 66302-206-04
- Packager: United Therapeutics Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TYVASO safely and effectively. See full prescribing information for TYVASO.
TYVASO® (treprostinil) inhalation solution, for oral inhalation use
Initial U.S. Approval: 2002RECENT MAJOR CHANGES
Warnings and Precautions (5.4) 05/2022 INDICATIONS AND USAGE
Tyvaso is a prostacyclin mimetic indicated for the treatment of:
- Pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%). (1.1)
- Pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%). (1.2)
DOSAGE AND ADMINISTRATION
- Use only with the Tyvaso Inhalation System. (2.1)
- Administer undiluted, as supplied. A single breath of Tyvaso delivers approximately 6 mcg of treprostinil. (2.1)
- Administer in 4 separate treatment sessions each day approximately 4 hours apart, during waking hours. (2.1)
- Initial dosage: 3 breaths (18 mcg) per treatment session. If 3 breaths are not tolerated, reduce to 1 or 2 breaths. (2.1)
- Dosage should be increased by an additional 3 breaths per treatment session at approximately 1- to 2-week intervals, if tolerated. (2.1)
- Titrate to target maintenance doses of 9 to 12 breaths per treatment session, 4 times daily. (2.1)
DOSAGE FORMS AND STRENGTHS
Sterile solution for oral inhalation: 2.9 mL ampule containing 1.74 mg treprostinil (0.6 mg per mL). (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Tyvaso may cause symptomatic hypotension. (5.1)
- Tyvaso inhibits platelet aggregation and increases the risk of bleeding. (5.2)
- Tyvaso dosage adjustments may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn. (5.3, 7.3)
- May cause bronchospasm: Patients with a history of hyperreactive airway disease may be more sensitive. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (≥4%) are cough, headache, nausea, dizziness, flushing, throat irritation, pharyngolaryngeal pain, diarrhea, and syncope. (6)
To report SUSPECTED ADVERSE REACTIONS, contact United Therapeutics Corp. at 1-866-458-6479 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
1.2 Pulmonary Hypertension Associated with ILD
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage in Adults
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Symptomatic Hypotension
5.2 Risk of Bleeding
5.3 Effect of Other Drugs on Treprostinil
5.4 Bronchospasm
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Bosentan
7.2 Sildenafil
7.3 Effect of Cytochrome P450 Inhibitors and Inducers
7.4 Effect of Other Drugs on Treprostinil
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Insufficiency
8.7 Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension (WHO Group 1)
14.2 Long-term Treatment of PAH
14.3 Pulmonary Hypertension Associated with ILD (WHO Group 3)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
Tyvaso is indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to improve exercise ability. Studies establishing effectiveness predominately included patients with NYHA Functional Class III symptoms and etiologies of idiopathic or heritable PAH (56%) or PAH associated with connective tissue diseases (33%).
The effects diminish over the minimum recommended dosing interval of 4 hours; treatment timing can be adjusted for planned activities.
While there are long-term data on use of treprostinil by other routes of administration, nearly all controlled clinical experience with inhaled treprostinil has been on a background of bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase type 5 inhibitor). The controlled clinical experience was limited to 12 weeks in duration [see Clinical Studies (14)].
1.2 Pulmonary Hypertension Associated with ILD
Tyvaso is indicated for the treatment of pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3) to improve exercise ability. The study establishing effectiveness predominately included patients with etiologies of idiopathic interstitial pneumonia (IIP) (45%) inclusive of idiopathic pulmonary fibrosis (IPF), combined pulmonary fibrosis and emphysema (CPFE) (25%), and WHO Group 3 connective tissue disease (22%) [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dosage in Adults
Tyvaso is intended for oral inhalation using the Tyvaso Inhalation System, which consists of an ultrasonic, pulsed delivery device and its accessories.
Tyvaso is dosed in 4 separate, equally spaced treatment sessions per day, during waking hours. Each treatment session will take 2 to 3 minutes. The treatment sessions should be approximately 4 hours apart.
Initial Dosage:
Therapy should begin with 3 breaths of Tyvaso (18 mcg of treprostinil) per treatment session 4 times daily. If 3 breaths are not tolerated, reduce to 1 or 2 breaths and subsequently increase to 3 breaths, as tolerated.
Maintenance Dosage:
Dosage should be increased by an additional 3 breaths per treatment session, 4 times daily at approximately 1- to 2-week intervals. Studies establishing effectiveness in patients with PAH and PH-ILD have used target doses of 9 to 12 breaths per treatment session, 4 times daily. If adverse effects preclude titration to target dose, Tyvaso should be continued at the highest tolerated dose.
If a scheduled treatment session is missed or interrupted, therapy should be resumed as soon as possible at the usual dose.
2.2 Administration
Tyvaso must be used only with the Tyvaso Inhalation System. Patients should follow the instructions for use for operation of the Tyvaso Inhalation System and for daily cleaning of the device components after the last treatment session of the day. To avoid potential interruptions in drug delivery because of equipment malfunction, patients should have access to a back-up Tyvaso Inhalation System device.
Do not mix Tyvaso with other medications in the Tyvaso Inhalation System. Compatibility of Tyvaso with other medications has not been studied.
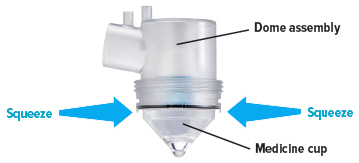
The Tyvaso Inhalation System should be prepared for use each day according to the instructions for use. One ampule of Tyvaso contains a sufficient volume of medication for all 4 treatment sessions in a single day. Prior to the first treatment session, the patient should twist the top off a single Tyvaso ampule and squeeze the entire contents into the medicine cup. Between each of the 4 daily treatment sessions, the device should be capped and stored upright with the remaining medication inside.
At the end of each day, the medicine cup and any remaining medication must be discarded. The device must be cleaned each day according to the instructions for use.
Avoid skin or eye contact with Tyvaso solution. Do not orally ingest the Tyvaso solution.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Symptomatic Hypotension
Treprostinil is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Tyvaso may produce symptomatic hypotension.
5.3 Effect of Other Drugs on Treprostinil
Co-administration of a cytochrome P450 (CYP) 2C8 enzyme inhibitor (e.g., gemfibrozil) may increase exposure (both Cmax and AUC) to treprostinil. Co-administration of a CYP2C8 enzyme inducer (e.g., rifampin) may decrease exposure to treprostinil. Increased exposure is likely to increase adverse events associated with treprostinil administration, whereas decreased exposure is likely to reduce clinical effectiveness [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
5.4 Bronchospasm
Like other inhaled prostaglandins, Tyvaso may cause acute bronchospasm. Patients with asthma or chronic obstructive pulmonary disease (COPD), or other bronchial hyperreactivity, are at increased risk for bronchospasm. Ensure that such patients are treated optimally for reactive airway disease prior to and during treatment with Tyvaso Inhalation Solution.
-
6 ADVERSE REACTIONS
The following potential adverse reactions are described in Warnings and Precautions (5):
- -
- Decrease in systemic blood pressure [see Warnings and Precautions (5.1)].
- -
- Bleeding [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pulmonary Arterial Hypertension
In a 12-week, placebo-controlled study (TRIUMPH I) of 235 patients with PAH (WHO Group 1 and nearly all NYHA Functional Class III), the most commonly reported adverse reactions on Tyvaso included cough and throat irritation, headache, gastrointestinal effects, muscle, jaw or bone pain, dizziness, flushing, and syncope. Table 1 lists the adverse reactions that occurred at a rate of at least 4% and were more frequent in patients treated with Tyvaso than with placebo.
Table 1: Adverse Events in ≥4% of PAH Patients Receiving Tyvaso and More Frequent* than Placebo in TRIUMPH I Adverse Event Treatment
n (%)Tyvaso
n=115Placebo
n=120- *
- More than 3% greater than placebo
Cough 62 (54) 35 (29) Headache 47 (41) 27 (23) Throat Irritation / Pharyngolaryngeal Pain 29 (25) 17 (14) Nausea 22 (19) 13 (11) Flushing 17 (15) 1 (<1) Syncope 7 (6) 1 (<1) The safety of Tyvaso was also studied in a long-term, open-label extension study in which 206 patients were dosed for a mean duration of 2.3 years, with a maximum exposure of 5.4 years. Eighty-nine percent (89%) of patients achieved the target dose of 9 breaths, 4 times daily. Forty-two percent (42%) achieved a dose of 12 breaths, 4 times daily. The adverse events during this chronic dosing study were qualitatively similar to those observed in the 12-week placebo-controlled trial.
In a prospective, observational study comparing patients taking Tyvaso (958 patient-years of exposure) and a control group (treatment with other approved therapies for PAH; 1094 patient-years), Tyvaso was associated with a higher rate of cough (16.2 vs. 10.9 per 100 patient-years), throat irritation (4.5 vs. 1.2 per 100 pt-years), nasal discomfort (2.6 vs. 1.3 per 100 pt-years), and hemoptysis (2.5 vs. 1.3 per 100 pt-years) compared to the control group.
6.2 Post-Marketing Experience
The adverse reaction of angioedema has been identified during the post-approval use of Tyvaso. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
7.1 Bosentan
In a human pharmacokinetic study conducted with bosentan (250 mg/day) and an oral formulation of treprostinil (treprostinil diolamine), no pharmacokinetic interactions between treprostinil and bosentan were observed.
7.2 Sildenafil
In a human pharmacokinetic study conducted with sildenafil (60 mg/day) and an oral formulation of treprostinil (treprostinil diolamine), no pharmacokinetic interactions between treprostinil and sildenafil were observed.
7.3 Effect of Cytochrome P450 Inhibitors and Inducers
In vitro studies of human hepatic microsomes showed that treprostinil does not inhibit cytochrome P450 (CYP) isoenzymes CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A. Additionally, treprostinil does not induce cytochrome P450 isoenzymes CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A.
Human pharmacokinetic studies with an oral formulation of treprostinil (treprostinil diolamine) indicated that co-administration of the cytochrome P450 (CYP) 2C8 enzyme inhibitor, gemfibrozil, increases exposure (both Cmax and AUC) to treprostinil. Co-administration of the CYP2C8 enzyme inducer, rifampin, decreases exposure to treprostinil. It is unclear if the safety and efficacy of treprostinil by the inhalation route are altered by inhibitors or inducers of CYP2C8 [see Warnings and Precautions (5.3)].
7.4 Effect of Other Drugs on Treprostinil
Drug interaction studies have been carried out with treprostinil (oral or subcutaneous) co-administered with acetaminophen (4 g/day), warfarin (25 mg/day), and fluconazole (200 mg/day), respectively, in healthy volunteers. These studies did not show a clinically significant effect on the pharmacokinetics of treprostinil. Treprostinil does not affect the pharmacokinetics or pharmacodynamics of warfarin. The pharmacokinetics of R- and S- warfarin and the international normalized ratio (INR) in healthy subjects given a single 25 mg dose of warfarin were unaffected by continuous subcutaneous infusion of treprostinil at an infusion rate of 10 ng/kg/min.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited case reports of treprostinil use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, there are risks to the mother and the fetus associated with pulmonary arterial hypertension (see Clinical Considerations). In animal studies, no adverse reproductive and developmental effects were seen for treprostinil at ≥9 and ≥145 times the human exposure when based on Cmax and AUC, respectively, following a single treprostinil dose of 54 mcg.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal reproduction studies have been conducted with treprostinil via continuous subcutaneous administration and with treprostinil diolamine administered orally. In studies with orally administered treprostinil diolamine, no adverse effect doses for fetal viability/growth, fetal development (teratogenicity), and postnatal development were determined in rats. In pregnant rats, no evidence of harm to the fetus was observed following oral administration of treprostinil diolamine at the highest dose tested (20 mg/kg/day), which represents about 154 and 1479 times the human exposure, when based on Cmax and AUC, respectively, following a single Tyvaso dose of 54 mcg. In pregnant rabbits, external fetal and soft tissue malformations and fetal skeletal malformation occurred. The dose at which no adverse effects were seen (0.5 mg/kg/day) represents about 9 and 145 times the human exposure, when based on Cmax and AUC, respectively, following a single Tyvaso dose of 54 mcg. No treprostinil treatment-related effects on labor and delivery were seen in animal studies. Animal reproduction studies are not always predictive of human response.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Clinical studies of Tyvaso did not include patients younger than 18 years to determine whether they respond differently from older patients.
8.5 Geriatric Use
Across clinical studies used to establish the effectiveness of Tyvaso in patients with PAH and PH-ILD, 268 (47.8%) patients aged 65 years and over were enrolled. The treatment effects and safety profile observed in geriatric patients were similar to younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant diseases or other drug therapy.
8.6 Patients with Hepatic Insufficiency
Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects with mild-to-moderate hepatic insufficiency. Uptitrate slowly when treating patients with hepatic insufficiency because of the risk of an increase in systemic exposure which may lead to an increase in dose-dependent adverse effects. Treprostinil has not been studied in patients with severe hepatic insufficiency [see Clinical Pharmacology (12.3)].
8.7 Patients with Renal Impairment
No dose adjustments are required in patients with renal impairment. Treprostinil is not cleared by dialysis [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Tyvaso is a sterile formulation of treprostinil, a prostacyclin mimetic, intended for administration by oral inhalation using the Tyvaso Inhalation System. Tyvaso is supplied in 2.9 mL low density polyethylene (LDPE) ampules, containing 1.74 mg treprostinil (0.6 mg/mL). Each ampule also contains 18.9 mg sodium chloride, 18.3 mg sodium citrate dihydrate, 0.58 mg sodium hydroxide, 11.7 mg 1 N hydrochloric acid, and water for injection. Sodium hydroxide and hydrochloric acid may be added to adjust pH between 6.0 and 7.2.
Treprostinil is (1R,2R,3aS,9aS)-[[2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]inden-5-yl]oxy]acetic acid. Treprostinil has a molecular weight of 390.52 and a molecular formula of C23H34O5.
The structural formula of treprostinil is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Treprostinil is a prostacyclin analogue. The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation.
12.2 Pharmacodynamics
In a clinical trial of 240 healthy volunteers, single doses of Tyvaso 54 mcg (the target maintenance dose per session) and 84 mcg (supratherapeutic inhalation dose) prolonged the corrected QTc interval by approximately 10 ms. The QTc effect dissipated rapidly as the concentration of treprostinil decreased.
12.3 Pharmacokinetics
Pharmacokinetic information for single doses of inhaled treprostinil was obtained in healthy volunteers in 3 separate studies. Treprostinil systemic exposure (AUC and Cmax) post-inhalation was shown to be proportional to the doses administered (18 mcg to 90 mcg).
Absorption
In a 3-period crossover study, the bioavailability of 2 single doses of Tyvaso (18 mcg and 36 mcg) was compared with that of intravenous treprostinil in 18 healthy volunteers. Mean estimates of the absolute systemic bioavailability of treprostinil after inhalation were approximately 64% (18 mcg) and 72% (36 mcg).
Treprostinil plasma exposure data were obtained from 2 studies at the target maintenance dose, 54 mcg. The mean Cmax at the target dose was 0.91 and 1.32 ng/mL with corresponding mean Tmax of 0.25 and 0.12 hr, respectively. The mean AUC for the 54-mcg dose was 0.81 and 0.97 hr∙ng/mL, respectively.
Distribution
Following parenteral infusion, the apparent steady state volume of distribution (Vss) of treprostinil is approximately 14 L/70 kg ideal body weight.
In vitro treprostinil is 91% bound to human plasma proteins over the 330 to 10,000 mcg/L concentration range.
Metabolism and Excretion
Of subcutaneously administered treprostinil, only 4% is excreted unchanged in urine. Treprostinil is substantially metabolized by the liver, primarily by CYP2C8. Metabolites are excreted in urine (79%) and feces (13%) over 10 days. Five apparently inactive metabolites were detected in the urine, each accounting for 10 to 15% of the dose administered. Four of the metabolites are products of oxidation of the 3-hydroxyloctyl side chain and 1 is a glucuroconjugated derivative (treprostinil glucuronide).
The elimination of treprostinil (following subcutaneous administration of treprostinil) is biphasic, with a terminal elimination half-life of approximately 4 hours using a 2-compartment model.
Specific Populations
Hepatic Insufficiency
Plasma clearance of treprostinil, delivered subcutaneously, was reduced up to 80% in subjects presenting with mild-to-moderate hepatic insufficiency. Treprostinil has not been studied in patients with severe hepatic insufficiency [see Use in Specific Populations (8.6)].
Renal Impairment
In patients with severe renal impairment requiring dialysis (n=8), administration of a single 1 mg dose of orally administered treprostinil pre- and post-dialysis resulted in AUC0-inf that was not significantly altered compared to healthy subjects [see Use in Specific Populations (8.7)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year rat carcinogenicity study was performed with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day. There was no evidence for carcinogenic potential associated with treprostinil inhalation in rats at systemic exposure levels up to 35 times the clinical exposure at the target maintenance dose of 54 mcg. In vitro and in vivo genetic toxicology studies did not demonstrate any mutagenic or clastogenic effects of treprostinil. Treprostinil sodium did not affect fertility or mating performance of male or female rats given continuous subcutaneous infusions at rates of up to 450 ng treprostinil/kg/min. In this study, males were dosed from 10 weeks prior to mating and through the 2-week mating period. Females were dosed from 2 weeks prior to mating until gestational day 6.
Oral administration of treprostinil diolamine to Tg.rasH2 mice at 0, 5, 10, and 20 mg/kg/day in males and 0, 3, 7.5, and 15 mg/kg/day in females daily for 26 weeks did not significantly increase the incidence of tumors.
Treprostinil diolamine was tested in vivo in a rat micronucleus assay and did not induce an increased incidence of micronucleated polychromatic erythrocytes.
13.2 Animal Toxicology and/or Pharmacology
In a 2-year rat study with treprostinil inhalation at target doses of 5.26, 10.6, and 34.1 mcg/kg/day, there were more deaths (11) in the mid- and high-dose treprostinil groups during the first 9 weeks of the study, compared to 1 in control groups. At the high-dose level, males showed a higher incidence of inflammation in teeth and preputial gland, and females showed higher incidences of inflammation and urothelial hyperplasia in the urinary bladder. The exposures in rats at mid- and high-dose levels were about 15 and 35 times, respectively, the clinical exposure at the target maintenance dose of 54 mcg.
-
14 CLINICAL STUDIES
14.1 Pulmonary Arterial Hypertension (WHO Group 1)
TRIUMPH I, was a 12-week, randomized, double-blind, placebo-controlled, multicenter study of patients with PAH. The study population included 235 clinically stable subjects with PAH (WHO Group 1), nearly all with NYHA Class III (98%) symptoms who were receiving either bosentan (an endothelin receptor antagonist) or sildenafil (a phosphodiesterase-5 inhibitor) for at least 3 months prior to study initiation. Concomitant therapy also could have included anticoagulants, other vasodilators (e.g., calcium channel blockers), diuretics, oxygen, and digitalis, but not a prostacyclin. These patients were administered either placebo or Tyvaso in 4 daily treatment sessions with a target dose of 9 breaths (54 mcg) per session over the course of the 12-week study. Patients were predominately female (82%), had the origin of PAH as idiopathic/heritable (56%), secondary to connective tissue diseases (33%) or secondary to HIV or previous use of anorexigens (12%); bosentan was the concomitant oral medication in 70% of those enrolled, sildenafil in 30%.
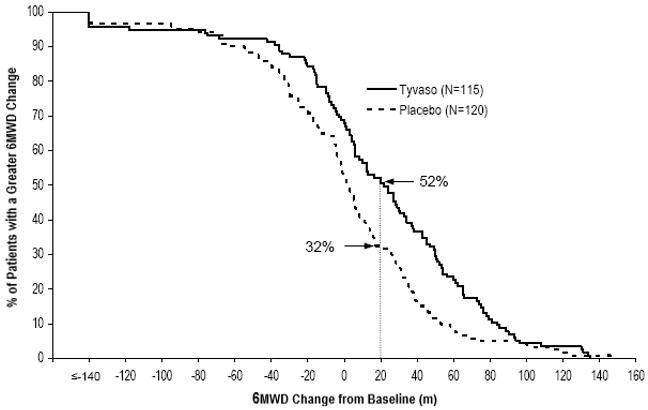
The primary efficacy endpoint of the trial was the change in 6-Minute Walk Distance (6MWD) relative to baseline at 12 weeks. 6MWD was measured at peak exposure (between 10 and 60 minutes after dosing), and 3 to 5 hours after bosentan or 0.5 to 2 hours after sildenafil. Patients receiving Tyvaso had a placebo-corrected median change from baseline in peak 6MWD of 20 meters at Week 12 (p<0.001). The distribution of these 6MWD changes from baseline at Week 12 were plotted across the range of observed values (Figure 1). 6MWD measured at trough exposure (defined as measurement of 6MWD at least 4 hours after dosing) improved by 14 meters. There were no placebo-controlled 6MWD assessments made after 12 weeks.
Figure 1: Distributions of 6MWD Changes from Baseline at Week 12 During Peak Plasma Concentration of Tyvaso
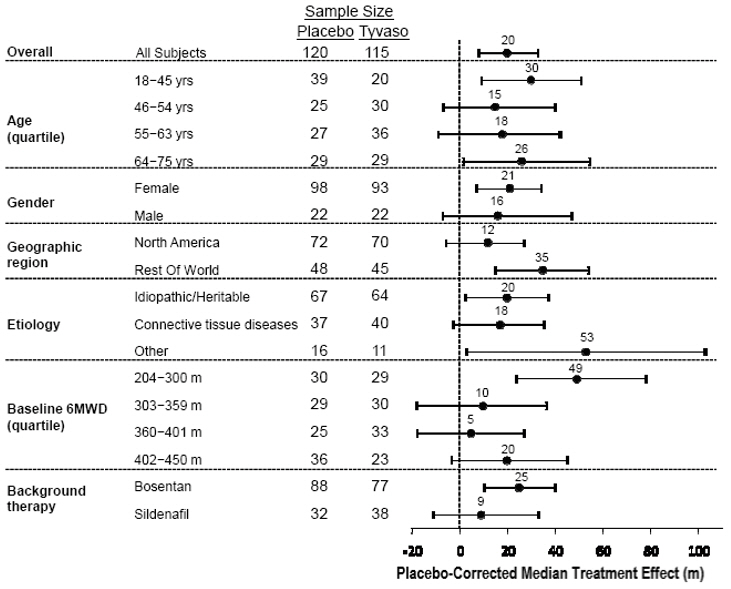
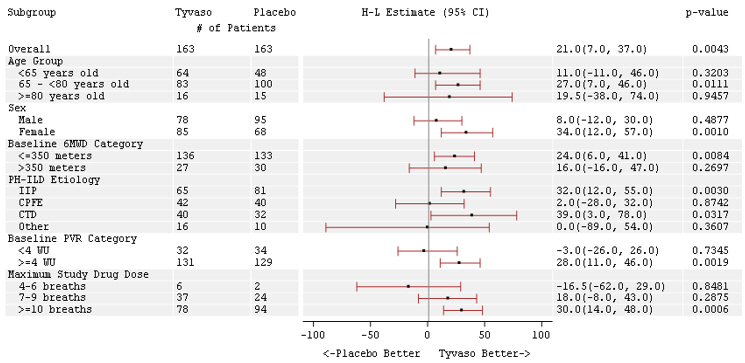
The placebo-corrected median treatment effect on 6MWD was estimated (using the Hodges-Lehmann estimator) within various subpopulations defined by age quartile, gender, geographic region of the study site, disease etiology, baseline 6MWD quartile, and type of background therapy (Figure 2).
Figure 2: Placebo-Corrected Median Treatment Effect (Hodges-Lehmann Estimate with 95% CI) on 6MWD Change from Baseline at Week 12 During Peak Plasma Concentration of Tyvaso for Various Subgroups
14.2 Long-term Treatment of PAH
In long-term follow-up of patients who were treated with Tyvaso in the pivotal study and the open-label extension (N=206), Kaplan-Meier estimates of survival at 1, 2, and 3 years were 97%, 91%, and 82%, respectively. These uncontrolled observations do not allow comparison with a control group not given Tyvaso and cannot be used to determine the long-term effect of Tyvaso on mortality.
14.3 Pulmonary Hypertension Associated with ILD (WHO Group 3)
INCREASE was a 16-week, randomized, double-blind, placebo-controlled, multicenter study that enrolled 326 patients with PH-ILD. Enrolled study patients predominately had etiologies of idiopathic interstitial pneumonia (45%) inclusive of idiopathic pulmonary fibrosis, combined pulmonary fibrosis and emphysema (25%), and WHO Group 3 connective tissue disease (22%). The mean baseline 6MWD was 260 meters.
Patients in the INCREASE study were randomized (1:1) to either placebo or Tyvaso in 4 daily treatment sessions with a target dose of 9 breaths (54 mcg) per session and a maximum dose of 12 breaths (72 mcg) per session over the course of the 16-week study. Approximately 75% of patients randomized to Tyvaso titrated up to a dose of 9 breaths, 4 times daily or greater, with 48% of patients randomized to Tyvaso reaching a dose of 12 breaths, 4 times daily during the study.
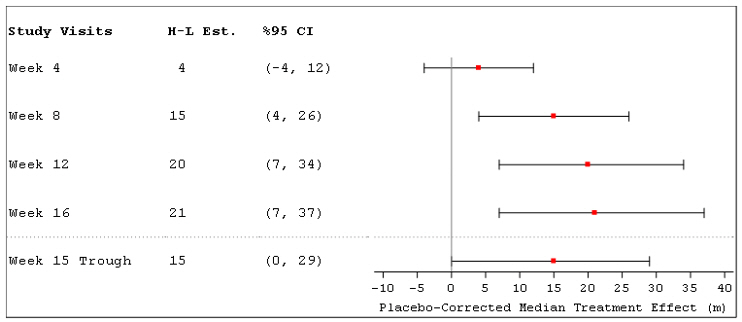
The primary efficacy endpoint was the change in 6MWD measured at peak exposure (between 10 and 60 minutes after dosing) from baseline to Week 16. Patients receiving Tyvaso had a placebo-corrected median change from baseline in peak 6MWD of 21 meters at Week 16 (p=0.004) using Hodges-Lehmann estimate (Figure 3).
Figure 3: Hodges-Lehmann Estimate of Treatment Effect by Visit for 6MWD at Peak Exposure (PH-ILD)
The treatment effect on 6MWD at Week 16 was consistent for various subgroups, including etiology of PH-ILD, disease severity, age, sex, baseline hemodynamics, and dose (Figure 4).
Figure 4: Forest Plot on Subgroup Analyses of Peak 6MWD (Meter) at Week 16 (PH-ILD)
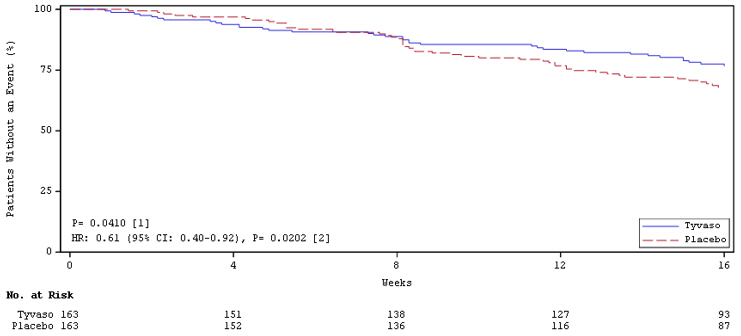
Time to clinical worsening in the INCREASE study was defined as the time of randomization until 1 of the following criteria were met: hospitalization due to a cardiopulmonary indication, decrease in 6MWD >15% from baseline directly related to PH-ILD at 2 consecutive visits and at least 24 hours apart, death (all causes), or lung transplantation. Treatment with Tyvaso in patients with PH-ILD resulted in numerically fewer hospitalizations. The numbers of reported deaths were the same for both treatment groups (Table 2). Overall, treatment with Tyvaso demonstrated a statistically significant increase in the time to first clinical worsening event (log-rank test p=0.041; Figure 5), and a 39% overall reduction in the risk of a clinical worsening event (HR=0.61 [95% CI; 0.40, 0.92]; Figure 5).
Table 2: Clinical Worsening Events (PH-ILD) Tyvaso
n=163
n (%)Placebo
n=163
n (%)HR (95% CI) Clinical worsening 37 (22.7%) 54 (33.1%) 0.61 (0.40, 0.92) First contributing event Hospitalization due to a cardiopulmonary indication 18 (11.0%) 24 (14.7%) Decrease in 6MWD >15% from baseline directly related to PH-ILD 13 (8.0%) 26 (16.0%) Death (all causes) 4 (2.5%) 4 (2.5%) Lung transplantation 2 (1.2%) 0 First of each event Hospitalization due to a cardiopulmonary indication 21 (12.9%) 30 (18.4%) Decrease in 6MWD >15% from baseline directly related to PH-ILD 16 (9.8%) 31 (19.0%) Death (all causes) 8 (4.9%) 10 (6.1%) Lung transplantation 2 (1.2%) 1 (0.6%) Figure 5: Kaplan-Meier Plot of Time to Clinical Worsening Events (PH-ILD)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tyvaso (treprostinil) inhalation solution is supplied in 2.9 mL clear LDPE ampules packaged as 4 ampules in a foil pouch. Tyvaso is a clear colorless to slightly yellow solution containing 1.74 mg treprostinil per ampule at a concentration of 0.6 mg/mL.
Ampules of Tyvaso are stable until the date indicated when stored in the unopened foil pouch at 20-25°C (68-77°F) with excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Once the foil pack is opened, ampules should be used within 7 days. Because Tyvaso is light-sensitive, unopened ampules should be stored in the foil pouch.
One ampule of Tyvaso should be used each day in the Tyvaso Inhalation System. After a Tyvaso ampule is opened and transferred to the medicine cup, the solution should remain in the device for no more than 1 day (24 hours). Any remaining solution should be discarded at the end of the day.
Tyvaso Inhalation System Starter Kit containing a 28-ampule carton of Tyvaso (7 foil pouches each containing four 2.9 mL ampules; each ampule contains 1.74 mg treprostinil [0.6 mg per mL]) and the Tyvaso Inhalation System. (NDC 66302-206-01)
Tyvaso Inhalation System Refill Kit containing a 28-ampule carton of Tyvaso (7 foil pouches each containing four 2.9 mL ampules; each ampule contains 1.74 mg treprostinil [0.6 mg per mL]) and accessories. (NDC 66302-206-02)
Tyvaso 4 Pack Carton with 1 foil pouch containing four 2.9 mL ampules. Each ampule contains 1.74 mg treprostinil (0.6 mg per mL). (NDC 66302-206-03)
Tyvaso Inhalation System Institutional Starter Kit containing a 4-ampule carton of Tyvaso (1 foil pouch containing four 2.9 mL ampules; each ampule contains 1.74 mg treprostinil [0.6 mg per mL]) and the Tyvaso Inhalation System. (NDC 66302-206-04)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Train patients in the administration process for Tyvaso, including dosing, Tyvaso Inhalation System set up, operation, cleaning, and maintenance, according to the instructions for use [see Dosage and Administration (2.1, 2.2)].
To avoid potential interruptions in drug delivery because of equipment malfunction, patients should have access to a back-up Tyvaso Inhalation System device [see Dosage and Administration (2.2)].
In the event that a scheduled treatment session is missed or interrupted, resume therapy as soon as possible [see Dosage and Administration (2.1)].
If Tyvaso comes in contact with the skin or eyes, instruct patients to rinse immediately with water [see Dosage and Administration (2.2)].
- SPL UNCLASSIFIED SECTION
-
INSTRUCTIONS FOR USE
TYVASO®
INHALATION SYSTEM
Instructions for UseTYVASO®
(treprostinil)
INHALATION
SOLUTIONTable of Contents
General Instructions 2 Introduction 3 Preparing for Treatment With TYVASO 3 Preparing the Use Environment for Treatment 3 Markings, Indicators, and Symbols 4 Gathering Supplies 6 Setting Up Your TYVASO Inhalation System 8 Filling the Inhalation Device Chamber and Medicine Cup 8 Assembling the Inhalation Device 10 Turning On the Inhalation Device 13 Setting the Number of Breaths in a Treatment Session 14 Inhaling Your Medicine (TYVASO) 16 Storing the TYVASO Inhalation System Between Treatment Sessions 20 Cleaning and Maintenance of the TYVASO Inhalation System 24 End of Day Cleaning of the Accessories 24 Weekly Cleaning 27 Monthly Refill Kit 27 Device Replacement 27 Charging Your TYVASO Inhalation System 28 Rechargeable Battery 28 Troubleshooting the TYVASO Inhalation System 30 Specifications 36 Glossary 38 Warranty Information 40 General Instructions
The TYVASO Inhalation System should be handled with care.
Follow these important instructions to ensure proper use:
- Always unplug the device after each use.
- Do not immerse the device in water or other liquids.
- Do not place the device in a microwave or regular oven.
- Do not place the device or use the device in the presence of strong electric or magnetic fields (eg, microwave oven, magnetic imaging equipment).
- Do not leave the device alone with a small child.
- Do not use the device near flammable liquids and materials or heated surfaces.
- Read the instructions carefully and completely to prevent damage to your TYVASO Inhalation System and help you get the best results.
- This device should only be used on the order of your doctor or licensed healthcare practitioner.
- Ensure the breath counter is correctly programmed prior to beginning a treatment (see page 14).
- Do not peel or remove the labels from device.
- Do not drop the device.
Introduction
Your doctor has prescribed TYVASO® (treprostinil) Inhalation Solution. Please see the accompanying Patient Package Insert for important safety information on TYVASO.
TYVASO is breathed in (inhaled) using the TYVASO Inhalation System, which consists of the inhalation device and its accessories.
These Instructions for Use (IFU) for the TYVASO Inhalation System provide important safety information. It is important that you read these instructions and the TYVASO Patient Package Insert (PPI) before setting up and using the TYVASO Inhalation System. If you have any questions, talk to your doctor or specialty pharmacy provider.
Before beginning treatment with TYVASO, you will receive either a Patient Starter Kit containing a 28-day supply of TYVASO or an Institutional Starter Kit containing a 4-day supply of medication. Both kits include two (2) complete inhalation devices (all accessories and supplies included). When you refill your prescription for TYVASO each month, you will receive a Refill Kit that contains a 28-day supply of TYVASO and new accessories. You will receive a replacement device every two (2) years.

Preparing for Treatment With TYVASO
Preparing the Use Environment for Treatment
Follow these important instructions before setting up your treatment:
- Use the device in a quiet, distraction-free area.
- Try to use the device at times when your treatment will not be interrupted. If you encounter any distractions during treatment, you can pause your treatment (see page 19).
- Use the device in a comfortable space where you can stand or sit in an upright position that allows you to take deep breaths.
- Use the device in an area that provides enough space for the TYVASO Inhalation System and its accessories.
- Gather all necessary supplies before starting to prepare for treatment (see pages 6 and 7).
- The TYVASO Inhalation System is recommended for use indoors. Be sure to use and store the inhalation device in environments that match the specified temperature and humidity ranges (see Specifications on page 36).
Markings, Indicators, and Symbols
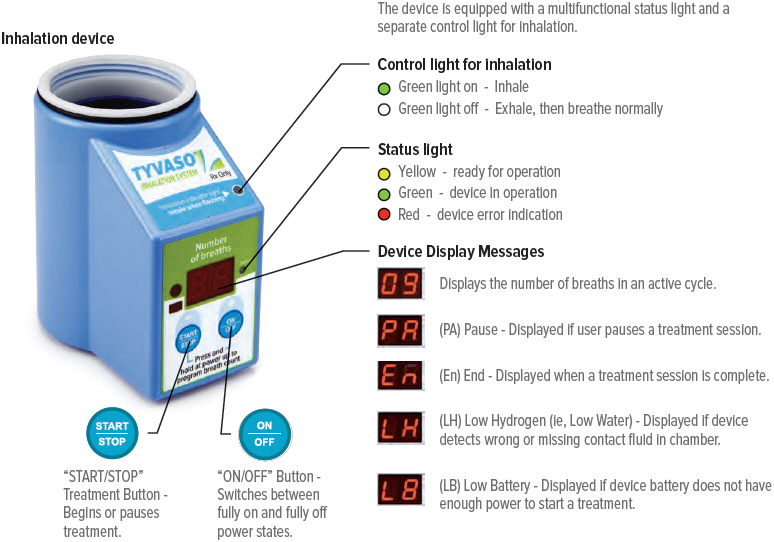
Device markings

The TYVASO Inhalation System complies with the requirements of Protection Class II. Class II equipment provides additional precautions, over and above basic insulation, to provide protection against electric shock. 
Please read the accompanying instructions and labels for important information regarding the TYVASO Inhalation System. 
The TYVASO Inhalation System has a Type B Applied part. Type B Applied parts comply with specific requirements to provide protection against shock and are not suitable for direct cardiac applications. 
The TYVASO Inhalation System should only be used on the order of your doctor or licensed healthcare provider. 
The TYVASO Inhalation System requires a 12V DC power supply. Gathering Supplies
Gather the following supplies before starting treatment. Use only the parts and accessories that are provided with your starter kit or in the monthly refill kit. Note: supplies are not shown to scale.
1. Inhalation device 2. Water Level Cup with 45 mL of distilled water 3. Pack of TYVASO ampules 


Use only distilled water to fill your inhalation device once per day. Use one (1) ampule per day. 4. One of the provided power sources 
Note: Supplies are not shown to scale. - *
- These accessories are replaced every month. Replacement accessories are included in the Monthly Refill Kit.
5. Accessories 




Medicine cups* Dome assembly with baffle plate inside* Inhalation piece* Mouthpiece* Two (2) Filter shells* 




Filter membranes (use two (2) per day)* Plugs* Treatment Tracker* Carrying case Distilled water carrier (optional) Setting Up Your TYVASO Inhalation System
Filling the Inhalation Device Chamber and Medicine Cup
Before using the TYVASO Inhalation System, you should:
- Wash your hands

- Make sure the device is NOT connected to a power source
- Make sure the device is resting on a stable, flat surface during assembly
ONLY USE DISTILLED WATER in the device. Distilled water is highly purified water that can be purchased at most grocery stores and pharmacies. It is necessary for the device to function properly. If you use another type of water (such as bottled or tap water), the device may not function properly.
1
Fill the white chamber inside the device with approximately 45 mL of distilled water (about 1.5 ounces), using the water level cup provided. Fill the cup until water level is between the two arrow markings.
There is a silver sensor on the inside wall of the chamber. The water level should be above the silver sensor and below the blue ring in the device chamber. DO NOT OVERFILL the chamber, or the medicine cup will not fit correctly. Check the water level after filling the chamber.
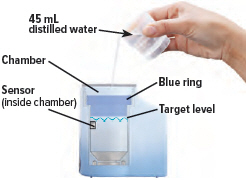
2
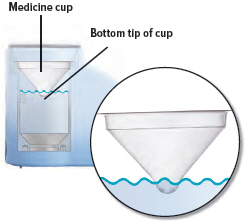
Obtain one (1) medicine cup and inspect it for any damage, holes, cracks, or dents. Do not use the medicine cup if it is damaged.Insert the empty medicine cup into the chamber of the device, making sure that the cup's bottom tip is in the distilled water. The cup will sit on the blue ring.

3
Carefully cut open the top of the foil pouch, making sure not to cut the ampules. Each pouch contains four (4) ampules.Remove one (1) ampule of TYVASO. Keep unused ampules in the foil pouch because the TYVASO medicine is sensitive to light.
One (1) ampule contains enough medicine for one (1) day of treatment no matter how many breaths your doctor has prescribed.


4
Gently hold the ampule in the upright (topup) position and twist off its top.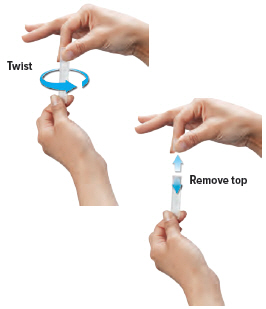

5
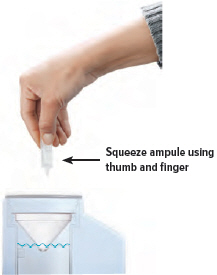
Point the ampule straight down toward the medicine cup's center to avoid spills.Gently squeeze the medicine out of the ampule into the medicine cup. Squeeze until it is empty. Check to see that all of the medicine is in the medicine cup.
Assembling the Inhalation Device
The TYVASO Inhalation System is designed so the parts only fit together properly one way.
When the device is assembled correctly, the parts should fit together easily.
Do not force the parts together.

1
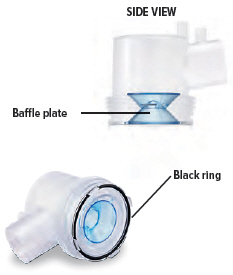
Visually check to make sure the blue plastic baffle plate and black ring are correctly placed in the dome assembly. They should appear as they do in the images below.In the unlikely event that the baffle plate is loose or disconnected, use a new dome assembly. If you need to order a new dome assembly, contact your specialty pharmacy provider.
2
Align the raised circle on the side of the dome assembly with the circle on the side of the device. Push down and screw the dome assembly onto the device clockwise (right) until you hear a click, indicating the dome assembly is fully connected to the medicine cup. When the dome assembly is properly aligned, the filter shell port will point to the back of the device.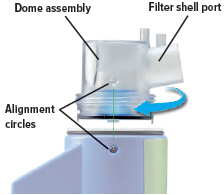

3
Insert the inhalation piece into the upper opening of the dome assembly and rotate toward the front of the device. Gently push down the inhalation piece to make sure it is securely inserted in the dome assembly.
4
Insert the mouthpiece into the inhalation piece.
5
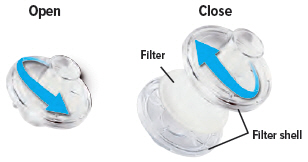
Each day you will need to use a new filter membrane in each filter shell.To install a new filter membrane:
- Open the filter shell by unscrewing the two (2) halves.
- Place a new filter membrane in one (1) of the filter shell halves.
- Close the filter shell by screwing the two (2) halves together until you can twist no further.
Note: New filter shells come with fresh filter membranes already installed.
6
Insert one (1) of the filter shells into the filter shell port on the side of the dome assembly and insert the other filter shell into the port on the bottom of the inhalation piece. The two (2) filter shells are identical and can be used in either opening.Make sure to insert the filter shells straight into their ports, rather than at an angle. If necessary, rotate the inhalation piece so you can insert the filter shell without the device getting in the way.

7
When the device is fully assembled, it should appear as it does in the photo below.For convenience, rotate the inhalation piece so you can best see the indicator lights and display screen, which provide important prompts during your treatment.
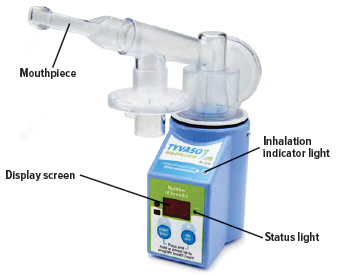
Turning On the Inhalation Device
1
Connect your device to one (1) of the power source options by plugging one end of the power cord into the back of the inhalation device and the other end into the power source.If using the AC wall plug, make sure to plug the output cable into the device before plugging adapter into the power source.
If using the rechargeable battery, make sure it is not also plugged into the AC wall plug.

2
To turn on the device, press and hold the ON/OFF button for approximately three (3) seconds. When you hear a short beep and the yellow status light is illuminated, release the ON/OFF button. Remember to press the center of the ON/OFF button when powering the device on or off.When the power is on, the display screen will show the last number of breaths programmed and a yellow light will appear next to the screen.
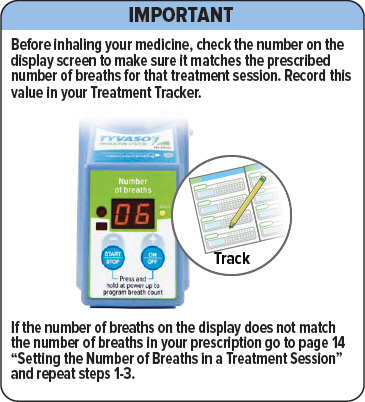
Make sure the number matches the prescribed number of breaths for that treatment session. If it does not match, see the next page for instructions on setting the number of breaths in a treatment session.

Setting the Number of Breaths in a Treatment Session
You will inhale TYVASO during four (4) treatment sessions each day. During each treatment session, you will take a series of breaths through the mouthpiece of the TYVASO Inhalation System.
Your doctor will prescribe the number of breaths you should inhale in each treatment session.
You should record the number of breaths in your Treatment Tracker.
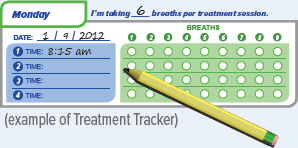
1
Make sure the device is powered off. To power off the device, press and hold the ON/OFF button for three (3) seconds. When you hear a short beep and the display screen turns off, release the ON/ OFF button.To set the number of breaths, press and hold both the START/STOP and ON/OFF buttons at the same time until the display flashes and the device emits three (3) short beeps.
The flashing display indicates the inhalation device is in breath programming mode. You cannot begin a treatment while the display is flashing.

2
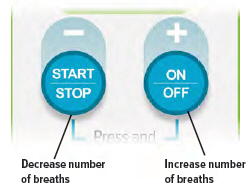
To increase the number of breaths press the + (ON/OFF) button.To decrease the number of breaths press the – (START/STOP) button.
Remember to press in the center of the buttons.
3
Once you have entered your prescribed number of breaths, remove your hands from the device and wait approximately 15 seconds.After approximately 15 seconds, the display will stop flashing and the device will emit three (3) short beeps, indicating that it has saved the new number of breaths. The new number of breaths will appear on the display.
Make sure the number of breaths on the display matches the number of breaths in your prescription. If the numbers do not match, repeat steps 1-3.

Inhaling Your Medicine, TYVASO® (treprostinil) Inhalation Solution
You will inhale TYVASO during four (4) treatment sessions each day. During each treatment session, you will take a series of breaths through the mouthpiece of the TYVASO Inhalation System.
Inhalation Tips
Technique:
When breathing each TYVASO treatment, be sure to keep the device level, directing the flow of medicine into the throat and not toward the roof of the mouth.
Seal your lips around the mouthpiece to ensure that you can inhale the full amount of TYVASO after it is produced by the device.
Inhalation:
Each breath should last approximately three (3) seconds, breathing "normal full breaths." Do not hold your breath. Exhale normally and prepare for the next breath.
1
Hold the device upright as shown below. Make sure you can see the display screen and lights clearly and that your hands do not cover the display screen or lights while holding device. If the inhalation piece or mouthpiece block your view of the display screen or lights, move the inhalation piece so you can best see them.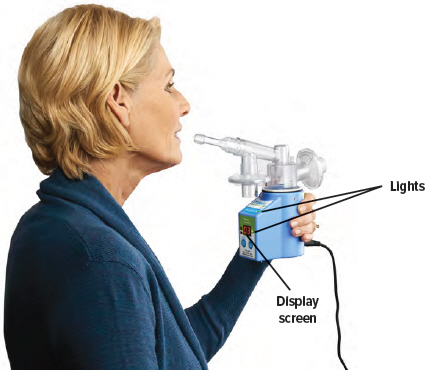
2
Perform steps A-G to complete one (1) treatment session.
Follow the instructions exactly to make sure you receive the correct medication dose.A 
B 
C 
D 
Press the START/STOP button to begin treatment. The status light turns green and the device emits two (2) short beeps. While the device emits one (1) long beep, exhale completely. After the device emits a short beep and while the inhalation indicator light is flashing green, place your lips securely around the mouthpiece and inhale slowly for the duration of the beep (at least three (3) seconds). Exhale normally, mark a circle in the Treatment Tracker and prepare for the next breath. 
E 
F 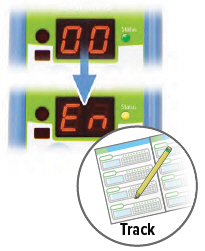
G 
Repeat steps B through D for the number of prescribed breaths and until the display counts down to "00". After the final breath, the display screen will show "00" then "En," indicating the treatment session is complete.
Check the number of breaths you inhaled on the Treatment Tracker.To turn off the device, press and hold the ON/OFF button for three (3) seconds and then release.
If the device is left in "En" mode for more than 60 seconds, it will turn off automatically.3
To pause the treatment between breaths, press the START/STOP button. The display screen will show "PA."
To resume treatment, press START/ STOP again. "PA" will disappear and the display screen will show the number of breaths remaining.
If the device is disconnected from its power source for any reason (for example, a power failure), reconnect the device to a power source. Follow the instructions on page 13 to turn on the device. The display will show how many breaths are left in that treatment session. Press the START/STOP button to continue your treatment session.
Storing the TYVASO Inhalation System Between Treatment Sessions
If you have more treatment sessions left in the day, perform the following steps.
If you have completed your last treatment session of the day, skip to Cleaning and Maintenance of the TYVASO Inhalation System (see page 24).
Be sure to pack all parts, including a backup power supply, in the carry case whenever transporting your device.
1
Disconnect the device from its power source.
2
Remove both filter shells.Do NOT remove the filter membranes from filter shells until after the last treatment session of the day.


3
Remove the mouthpiece.
4
Remove the inhalation piece.
5
Leave the dome assembly and medicine cup (with the medicine still in it) connected to the device.
6
Insert a plug into each of the open holes on the dome assembly to prevent the medicine from spilling out.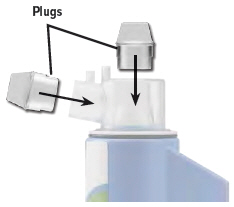

7
The inhalation device with the plugged dome assembly and all the accessories can be stored in the carrying case between treatment sessions.

Cleaning and Maintenance of the TYVASO Inhalation System
End of Day Cleaning of the Accessories
1
Disconnect the device from the power source.
2
Remove both filter shells.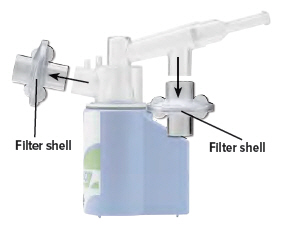
3
Open the filter shells by twisting in opposite directions.Remove and discard the used filter membranes.

4
Remove the mouthpiece.
5
Remove the inhalation piece.
6
Remove the dome assembly by turning it counter-clockwise (to the left). The medicine cup should stay attached to the dome assembly.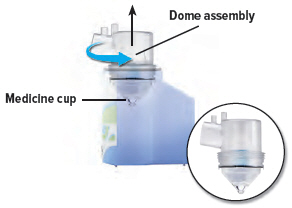

7
Remove the medicine cup by gently squeezing on the sides where it is attached to the dome assembly. Empty the medicine cup into a sink or waste basket, then discard the medicine cup.Be careful not to spill the medicine when removing or discarding the medicine cup.

8
Empty the distilled water from the chamber and let the inhalation device air dry upside down. You can wipe the chamber with a soft cloth or paper towel to collect water.
9
Clean the accessories (pictured below) by hand in mild, soapy, warm water, then rinse them thoroughly with water. Allow accessories to air dry.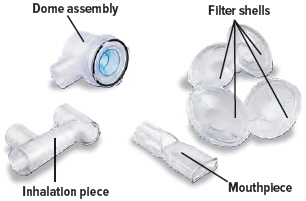

10
Once all the items are dry, the filter shells, inhalation piece, mouthpiece, dome assembly, and inhalation device can be stored in the carrying case until the next day's treatment sessions.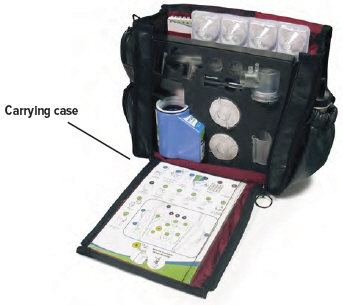
Weekly Cleaning
- Once a week, use a clean cloth to wipe the interior of the inhalation device chamber, be sure to wipe the disc in the bottom of the device. You may wipe the exterior of the device with a damp cloth if the lights or buttons become difficult to see. Proper cleaning will help to avoid corrosion and leaks and keep your device working properly.
Monthly Refill Kit
- Once a month, you will receive a refill kit that will come with a new set of accessories from your specialty pharmacy provider. Inspect the shipment to be sure all parts are included (see page 7). Once the new kit has arrived, discard the used dome assembly, inhalation piece, mouthpiece, and two (2) filter shells. Do not recycle the used accessories.
Device Replacement
- The inhalation device should be replaced every two (2) years from your first day of use. Replacement inhalation devices will be supplied by your specialty pharmacy provider.

Charging Your TYVASO Inhalation System
Rechargeable Battery
The rechargeable battery is supplied with its own instructions for use. Refer to these instructions for more information.
- Your TYVASO Inhalation System is supplied with a portable, rechargeable battery. Do NOT use other batteries.

- Your battery can only be charged by using one (1) of the AC wall plugs that comes with your inhalation device.

- To check the battery's voltage level, press and hold the blue button on the front of the battery. You can only check the battery's voltage level when the battery is not plugged into a power source.
The light below the button will turn on and indicate the battery's voltage level. A red light indicates the battery needs to be charged. A green light indicates the battery is ready to operate the device.

- To charge the battery, connect the AC wall plug to the battery pack and plug the AC wall plug into an outlet.A yellow light on the front of the battery will turn on, indicating that the battery is charging.

- Your battery may take up to 40 hours to fully charge. It is not possible to use the TYVASO Inhalation System with the battery while it is recharging.
A fully charged battery will typically last up to 200 inhalations. A battery charged for one (1) night (about 10 hours) will typically last up to one (1) day of therapy (about 40 inhalations).

- You should connect the charged battery to the TYVASO Inhalation System only for your treatment. When using the battery pack to power the device, make sure the battery is not plugged into a power source.

- After your treatment session is complete, remove the power plug of the battery from the device.
- Charge the battery whenever you are not using it to power the inhalation device.
- Always have your AC wall plug or 12V DC adapter available for backup, in case the battery is not charged.

Troubleshooting the TYVASO Inhalation System
Problem Possible causes Corrective actions Low battery (LB) 
- Low battery
- Unplug the battery from the device. Charge the battery by attaching it to the AC wall plug and outlet.
- Use an alternative power source, such as the AC wall plug or 12V DC adapter, for treatment.
- Defective adapter (AC wall plug or 12V DC adapter)
- Ensure that the plug is properly connected to an outlet.
- AC wall plug is still plugged into battery and battery is connected to device.
- Turn off device (see page 14).
- Disconnect AC wall plug from battery.
- Check battery status, following instructions on page 28.
- If device is properly powered and still does not function, contact your specialty pharmacy provider for a replacement.
Low hydrogen (LH) 
- Device chamber is empty or distilled water level in the device chamber is too low.
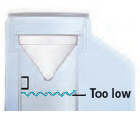
- Unplug the device from its power source.
Remove the dome assembly carefully, making sure not to spill its medicine. Empty the device chamber then refill it with distilled water (see page 8) and reconnect the dome assembly (see page 10).
- The distilled water you are using is too purified
- Empty device chamber. Add one (1) teaspoon of tap water to the water fill cup. Fill cup with distilled water up to level between the two arrow markings on cup (see page 8). Pour cup's contents into device chamber.
- The sensor has a thin layer of build-up
- Clean sensor and interior surfaces of water chamber with a clean cloth.
- If device is properly powered and still does not function, contact your specialty pharmacy provider for a replacement.
No medicine comes out of the device during a treatment session - No TYVASO® (treprostinil) Inhalation Solution in the medicine cup

- Unplug the device from its power source. Fill medicine cup with one (1) ampule of TYVASO.
- Damaged medicine cup

- Unplug the device from its power source. Replace the medicine cup.
- Distilled water level in the device chamber is too high
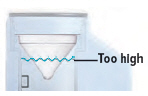
- Unplug the device from its power source. Empty the device chamber then refill it with 45 mL of distilled water.

- Multiple medicine cups attached to the dome assembly

- Unplug the device from its power source. Remove and dispose of all medicine cups in the device. Insert a single, new medicine cup into device chamber and fill with one (1) ampule of TYVASO.
Difficult to breathe in medicine through the mouthpiece - Filter membrane is clogged
- Unplug the device from its power source. Replace filter membrane (see page 11).

No "click" was heard when attaching the dome assembly - No medicine cup in the chamber of the device
- Insert an empty medicine cup into the chamber of the device and fill it with one (1) ampule of TYVASO.
- Multiple medicine cups attached to the dome assembly

- Unplug the device from its power source. Remove and dispose of all medicine cups in the device. Insert a single, new medicine cup into device chamber and fill with one (1) ampule of TYVASO.
- Dome assembly is not securely in place

- Unplug the device from its power source. Align the circle on the side of the dome assembly with the circle on the side of the device. Push down and screw the dome assembly onto the device clockwise (right) until you hear a click, indicating the dome assembly is fully connected to the medicine cup.
Loss of power during treatment - Device is disconnected from its power source
- Reconnect the device to a power source and press the ON/OFF button to turn on the device. The display will show how many breaths are left in that treatment session. Press the START/STOP button to continue your treatment session.
- Power source is temporarily disrupted (for example, electricity interruption due to a storm)
- Reconnect the device to a power source and turn on the device (see page 13). The display will show how many breaths are left in that treatment session. Press the START/ STOP button to continue your treatment session.
Inhalation Device
Model TD-100/A Size 98 × 66 × 105 mm Weight, inhalation device 280 g (9.9 ounces) Types of power supply AC wall plug
12V DC adapter
Rechargeable batteryPower input 12V DC, 1.5A maximum Operating power consumption 18 Watt maximum Ultrasonic frequency 2.4 MHz (nominal) Nebulization rate 0.50 - 0.55 mg/min (0.9% Saline) Medicine cup capacity 6 mL, nominal Contact fluid chamber capacity 45 mL, nominal Electric protection class II Type B Storage temperature/humidity -5 to 40°C/20-80% relative humidity Operating temperature/humidity 15 to 25°C/40-75% relative humidity Packaging Dimensions (Approximate Length × Width × Height)
Patient Starter Kit (PSK) 12.2" × 14.3" × 16.0" Monthly Refill Kit (MRK) 9.9" × 6.1" × 16.1" Institutional Starter Kit (ISK) 12.2" × 14.3" × 16.0" TYVASO Mass and Particle Specifications for 9 breaths
Mass Median Aerosol Diameter (MMAD)* mean = 2.0 μm
SD = 0.3Total Emitted Dose per Breath† mean = 6.0 μg
SD = 0.4Total Aerosol Mass* mean = 58 μg
SD = 5.9Total Respirable Dose* mean = 44.6 μg
SD = 3.5Respirable Fraction* mean = 73%
SD = 5%Geometric Standard Deviation (GSD)* mean = 2.6 μm
SD = 0.4Accessories
Note: Part number subject to change. ON-110HPA Rechargeable battery ON-100Z 12V DC adapter ON-100N-US AC wall plug ON-102/1/C Medicine cup, Quantity-16 ON-109 Filter membranes ON-120/C Plugs ON-101/C Filter shell TD-103/C Dome assembly with baffle plate ON-104/C Inhalation piece ON-105/C Mouthpiece TD-118 Water level cup TD-153 Carrying case TD-155 Distilled water carrier Glossary
Accessories: Parts of the TYVASO Inhalation System. See pages 6 and 7.
Ampule: A sealed, lightweight clear plastic vial containing a 1-day supply of TYVASO® (treprostinil) Inhalation Solution.
Control light for inhalation: A green LED on the top surface of the inhalation device that signals when you should inhale.
Baffle plate: A blue plastic piece that is inside the dome assembly. The baffle plate helps turn TYVASO into particles that are the correct size to inhale.
Black ring: A round seal that fits on the bottom of the dome assembly. The seal helps ensure that TYVASO does not mix with the distilled water in the device chamber.
Display screen: A small area on the inhalation device that displays number and letter prompts to guide you through your treatment sessions.
Distilled water: Water that is highly purified so that it contains only essential elements.
Dome assembly: The plastic accessory that contains the baffle plate and connects the mouthpiece, inhalation piece, and filter shells to the base of the inhalation device.
Filter membrane: The white pad that goes into the filter shells.
Filter shells: Plastic accessories that hold the filter membranes.
Inhalation piece: The plastic accessory that connects the mouthpiece with the dome assembly.
Inhalation device: The base of the TYVASO Inhalation System to which the accessories connect. The inhalation device contains the display screen and lights.
Inhale: How you will breathe in TYVASO with the TYVASO Inhalation System.
Medicine cup: The disposable plastic cone-shaped cup into which TYVASO is poured. The medicine cup fits inside the inhalation device chamber.
Mouthpiece: The plastic part that you will breathe through (using your mouth) to inhale TYVASO.
ON/OFF button: A manually activated control on the front of the device that switches between fully on and fully off power states.
Plugs: Plastic accessories that are inserted into the openings of the dome assembly between treatment sessions. Plugs help keep TYVASO from spilling if the inhalation device tips over.
Prompts: The audio and visual signals that help guide you through the treatment sessions.
Sensor: The silver object on the inside wall of the device chamber. The sensor must be covered with distilled water for the TYVASO Inhalation System to function properly.
Status light: A multicolored LED on the front of the inhalation device that indicates the device's operational status.
Start/Stop treatment button: A manually activated control on the front of the device that begins or pauses treatment.
Specialty pharmacy provider: A pharmacy that carries only specialized medicines and medical devices. Your specialty pharmacy provider is a good source of information about TYVASO and the TYVASO Inhalation System.
Treatment session: One (1) of four (4) daily sessions during which you will take TYVASO with a specific number of inhalations.
TYVASO: The prescription medicine that you will use with the TYVASO Inhalation System.
Water chamber: The white hollow portion in the center of the inhalation device into which distilled water and the medicine cup are placed.
Warranty Information
Your TYVASO Inhalation System is granted a full replacement or repair warranty good for two (2) years from your date of receipt of the TYVASO Inhalation System Starter Kit or five (5) years from the date of manufacture, whichever comes first. This warranty applies to the TYVASO Inhalation System device only. Accessory components are not covered under warranty.
Circumstances that may void your warranty include:
- Modification or disassembly of the TYVASO Inhalation System device by anyone other than a factory-authorized technician
- Failure to comply with the written Instructions for Use manual when operating the TYVASO Inhalation System
- Unapproved use of the TYVASO Inhalation System
For all inquiries relating to service or warranty for your TYVASO Inhalation System, contact your specialty pharmacy provider. You should have the following information available:
- Device serial number (located on bottom of TYVASO Inhalation System)
- Date TYVASO Inhalation System was acquired
- Nature of the problem and any steps taken to fix it
Graphics in IFU are for representation only. Images may not be shown to scale
TYVASO®
INHALATION SYSTEM
Instructions for UseTYVASO Inhalation Solution is for prescription use only.
TYVASO is a registered trademark of United Therapeutics Corporation.
Literature Issued 2016-07-30
For further questions and information, or to report an adverse reaction, please call 1-877-UNITHER (1-877-864-8437).
Emergency contact information
Clinician: ___________________________________
Nurse educator: ______________________________
Specialty pharmacist: __________________________
United Therapeutics: ___________________________
Distributed by:
United Therapeutics Corporation
Research Triangle Park, North Carolina 27709TYVASO®
(treprostinil)
INHALATION
SOLUTION© 2016, United Therapeutics Corporation. All rights reserved.
RTP-1-00052 Rev. E -
INSTRUCTIONS FOR USE
TYVASO®
INHALATION SYSTEMInstructions for Use Manual
TYVASO®
(treprostinil)
INHALATION
SOLUTIONContents
Overview of your TYVASO Inhalation System 4 Introduction 6 Safety and general instructions 8 Buttons, indicators, and markings 10 Inhalation device display screens 16 Programming your TYVASO Inhalation System before use 18 Charging device before use 20 Setting your prescribed dose 22 Adjusting device's audio volume 24 Preparing and using your TYVASO Inhalation System for daily treatments 26 Prepare a proper environment 28 Gather supplies 29 Fill water chamber and medicine cup 32 Assemble inhalation device 35 Power on inhalation device 40 Inhale your medicine 42 Cleaning and Storing your TYVASO Inhalation System 46 Storing between sessions during the day 48 End of day cleaning 52 Recharging the battery 57 Weekly cleaning 59 Monthly Refill Kit 60 Replacing your devices 61 Help / More information about your TYVASO Inhalation System 62 Troubleshooting 64 Specifications 76 Electromagnetic compatibility (EMC) 79 Glossary 87 Warranty information 90 Overview of your TYVASO Inhalation System
Section overview
This section introduces you to your TYVASO Inhalation System and provides important safety information about using your system.
What you will need: ▶ A clean place to review these instructions ▶ TYVASO Inhalation System to refer to while reading instructions What is covered in this section: A: Introduction 6 B: Safety and general instructions 8 C: Buttons, indicators, and markings 10 D: Inhalation device display screens 16 Important:
Do not start treatment with TYVASO until you have been trained to use the TYVASO Inhalation System. Make sure you understand all of the directions. Always ask your doctor or specialty pharmacy provider if you have any questions or are unsure of anything you are taught.
A: Introduction Your doctor has prescribed TYVASO® (treprostinil) Inhalation Solution. Please see the accompanying Patient Information for important safety information on TYVASO.
TYVASO is a prescription medicine used in adults to treat pulmonary arterial hypertension (PAH; WHO Group 1) and pulmonary hypertension associated with interstitial lung disease (PH-ILD; WHO Group 3), which are diseases causing high blood pressure in the arteries of your lungs. TYVASO can improve exercise ability. The effects decrease over 4 hours; treatment timing can be adjusted for planned activities.
TYVASO is breathed in (inhaled) using the TYVASO Inhalation System, which consists of the inhalation device and its accessories.
This Instructions for Use manual for the TYVASO Inhalation System provides important safety information. It is important that you read these instructions and the TYVASO Patient Information before setting up and using the TYVASO Inhalation System. If you have any questions, talk to your doctor or specialty pharmacy provider.
Before beginning treatment with TYVASO, you will receive either a Patient Starter Kit containing a 28-day supply of TYVASO or an Institutional Starter Kit containing a 4-day supply of medication.
Both kits include 2 complete inhalation devices (all accessories and supplies included). When you refill your prescription for TYVASO each month, you will receive a Refill Kit that contains a 28-day supply of TYVASO and new accessories. You will receive replacement devices every 2 years from your date of receipt of the TYVASO Inhalation System.
 CAUTION: Federal law restricts this device to sale by or on the order of a physician, or other licensed practitioner.
CAUTION: Federal law restricts this device to sale by or on the order of a physician, or other licensed practitioner.Important:
- Keep this Instructions for Use manual in a safe place where you can easily get to it for reference. For example, store the booklet in the TYVASO Inhalation System carrying case, along with your other supplies.
- TYVASO Inhalation System is intended solely for the delivery of TYVASO (treprostinil) Inhalation Solution. TYVASO is for administration only with the TYVASO Inhalation System.
B: Safety and general instructions The TYVASO Inhalation System should be handled carefully. Take the following precautions and follow all instructions in this document to avoid injury and ensure proper use:
Delivering treatments:
- Read the instructions carefully and completely to prevent damage to your TYVASO Inhalation System and help you get the best results.
- This device should only be used on the order of your doctor or licensed healthcare practitioner.
- Conduct only the number of treatment sessions and inhalations you have been prescribed.
- Ensure the breath counter is correctly programmed prior to beginning a treatment (see page 22).
- Turn off the device when not in use.
- Do not use the device with an anesthetic breathing system or ventilator breathing system.
- Use only the supplies provided in the Starter Kit and Monthly Refill Kit for correct device function.
Handling the device:
- Do not peel or remove the labels from the device.
- Do not drop the device.
- The device does not include internal, replaceable parts. Do not attempt to open the device, modify the device, or remove device labeling.
Your environment:
- Do not leave the device alone with a small child.
- Do not immerse the device in water or other liquids, or place in dishwasher.
- Do not place any system components in a microwave, conventional oven, or dishwasher.
- Do not use the device near flammable liquids and materials or heated surfaces.
- Do not place the device or use the device in the presence of strong electric or magnetic fields (e.g., microwave oven, magnetic imaging equipment).
- Wireless communications equipment (e.g., cell phone) can affect operation of the device and should be kept at least a distance of 3.3 meters (about 11 feet) away while using the device.
- If the device performance is affected by exposure to any conditions listed here, see the Troubleshooting section, or contact your healthcare provider or specialty pharmacy provider.
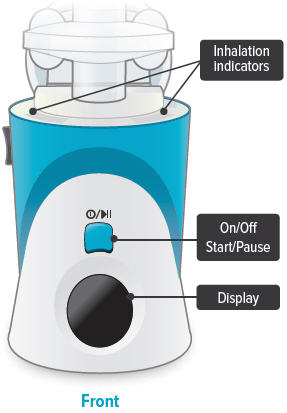
C: Buttons, indicators, and markings Inhalation device
Inhalation indicator lights
Lights on top of device flash green when you should inhale.
 On/Off, Start/Pause (blue) button
On/Off, Start/Pause (blue) button
Press and hold to power device on or off. Once device is on, press and immediately release (do not hold down) to start or pause treatment.
Device Display
Provides instructions and device information.
Run / Program switch
Slide up to Run mode when you are ready to deliver your dose. Slide down to Program mode to program the number of breaths for your dose.
Volume / Breaths toggle button
When set to Run mode, push + to increase beeping volume, or push - to decrease beeping volume.
When set to Program mode, push + to increase the number of breaths, or push - to decrease the number of breaths required for each dose.
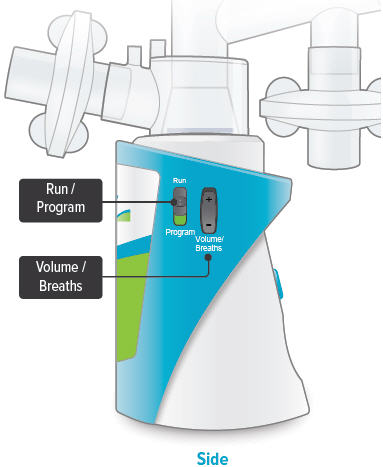
Power status light
 Lights green when power is connected and battery is charging.
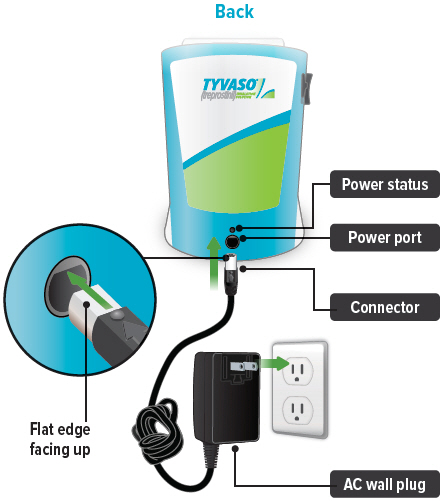
Lights green when power is connected and battery is charging.Power port
Port for plugging into a power source using the AC wall plug.

Additional device markings 
Manufacturer. Indicates the medical device manufacturer.
(Symbol 5.1.1 of ANSI/AAMI/ISO 15223-1: 2012 Medical devices - symbols to be used with medical devices labels, labeling, and information to be supplied - part 1: general requirements)
Equipment should not be disposed of in the trash.
(Figure 1 of BS EN 50419:2006 - Marking of Electrical and Electronic Equipment in accordance with Article 11(2) of Directive 2002/96/EC (WEEE))
Catalogue number. Indicates the manufacturer's catalogue number so that the medical device can be identified. (Symbol 5.1.6 of ANSI/AAMI/ISO 15223-1: 2012 Medical devices - symbols to be used with medical devices labels, labeling, and information to be supplied - part 1: general requirements) 
Serial number. Indicates the manufacturer's serial number so that a specific medical device can be identified. (Symbol 5.1.7 of ANSI/AAMI/ISO 15223-1: 2012 Medical devices - symbols to be used with medical devices labels, labeling, and information to be supplied - part 1: general requirements) 
Consult instructions for use. Please read the accompanying instructions and labels for important information regarding the TYVASO Inhalation System. (Symbol 5.4.3 of ANSI/AAMI/ISO 15223-1: 2012 Medical devices - symbols to be used with medical devices labels, labeling, and information to be supplied - part 1: general requirements) 
The TYVASO Inhalation System has a Type BF Applied part. Type BF Applied parts comply with specific requirements to provide protection against shock and are not suitable for direct cardiac applications.
(Symbol 5333 of IEC 60417 Database Snapshot - Graphical symbols for use on equipment)
The TYVASO Inhalation System requires a 14V DC power supply. Use only the power supply intended for the TYVASO Inhalation System.
(Direct Current, Symbol 5031 of IEC 60417 Database Snapshot - Graphical symbols for use on equipment)
The TYVASO Inhalation System complies with the requirements of Protection Class II. Class II equipment provides additional precautions, over and above basic insulation, to provide protection against electric shock. (Class II equipment, Symbol 5172 of IEC 60417 Database Snapshot - Graphical symbols for use on equipment) 
The TYVASO Inhalation Device provides level 2 solid particle protection and level 2 liquid ingress protection per IEC 60529 specifications. 
The TYVASO Inhalation System should only be used on the order of your doctor or licensed healthcare provider. (Symbol statement as provided under 21 CFR 801.109(b)(1)) 
Power stand by. Indicates the control for powering on and off the TYVASO Inhalation System. ("On" / "Off", Symbol 5010 of IEC 60417 Database Snapshot - Graphical symbols for use on equipment) 
Start/Pause. Indicates the control for starting a treatment session once the device is powered on, and for pausing a treatment once a treatment session has started. (Play and Pause, Symbols 5107B and 5111B, respectively, of IEC 60417 Database Snapshot - Graphical symbols for use on equipment) D: Inhalation device display screens 



Splash screen
Device name and software versionLast Treatment
Time since your last treatmentProgram Breaths
Number of breaths set in Program modeAdjust Volume
Audio volume level set in Run mode



Breaths Left
Number of breaths left in a current doseExhale
Prompt to exhale during a doseInhale
Prompt to inhale during a doseDone
Treatment session is complete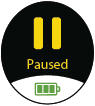

Pause
You have paused a treatment sessionCall Support
Device is not working, call your specialty pharmacy provider for support

Add Water
Wrong or missing fluid in water chamberCharge Battery
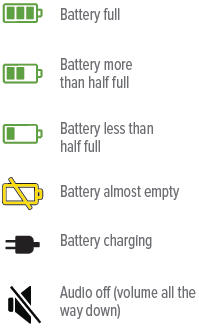
Battery not charged enough to deliver treatmentStatus icons
Icons that might appear at bottom of the screenProgramming your TYVASO Inhalation System before use
Section overview
This section provides instructions for charging your device, setting your dose, and adjusting the device's audio volume before you use the device for a treatment.
What you will need: ▶ A clean place to work with the device ▶ TYVASO Inhalation Device ▶ The number of breaths your doctor prescribed for each dose What is covered in this section: A: Charging device before use 20 B: Setting your prescribed dose 22 C: Adjusting device's audio volume 24 Important:
Do not start treatment with TYVASO until you have been trained to use the TYVASO Inhalation System. Make sure you understand all of the directions. Always ask your doctor or specialty pharmacy provider if you have any questions or are unsure of anything you are taught.
A: Charging device before use 1. Plug in device
Important: A new device might not be fully charged when you receive it. Always charge the device before you first use it. You can also charge the device overnight, when not in use and in between uses.
Plug the AC wall plug's white connector into the port on the back of the inhalation device. Then, plug the AC wall plug into the wall outlet.
The power status light above the port will light green when properly plugged in.
2. Check the battery's status
Make sure the Run / Program switch is set to Run. Press and hold the blue button to power on the device.
The battery icon at the bottom of the screen indicates battery status.
When you are done checking the battery status, press and hold the On/Off button until the display screen shuts off (note: letting the button go before the screen shuts off will start a treatment session).
If there is not enough charge to conduct a treatment session, "Charge battery" appears on screen. 

B: Setting your prescribed dose Your doctor will prescribe the number of breaths you should take in each treatment session. You should program this number into the inhalation device before you use the device.
1. Switch to Program
Slide the Run / Program switch on the side of the device down to Program mode. In Program mode you enter the prescribed number of breaths for each dose. You cannot begin a treatment in Program mode.
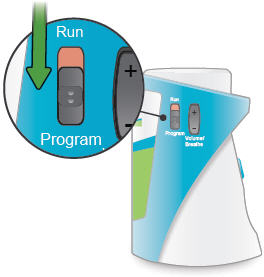
2. Power on
Press and hold the On/Off button until the display screen turns on. The Program Breaths screen appears. The number of breaths currently set for each treatment session will flash.
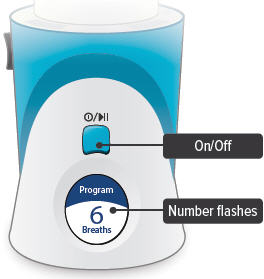
3. Set breaths
Use the Volume / Breaths toggle button to enter your prescribed number of breaths onto the program screen.
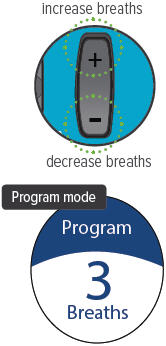
4. Switch to Run
Slide the Run / Program switch up to Run mode. Make sure your new breath count appears on screen.
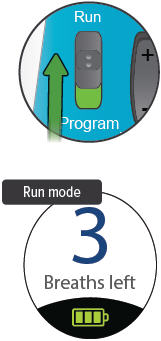
5. Power off
Press and hold the On/Off button until the display screen shuts off (note: letting the button go before the screen shuts off will start a treatment session).
Note: You will not need to program the breath count again, unless your prescribed number of breaths changes.

C: Adjusting device's audio volume You can use the Volume / Breaths toggle button to adjust the volume of the audible signals (beeps) that the device provides as feedback during treatment sessions.
1. Switch to Run
Slide the Run / Program switch up to Run mode, if it is not in this position already.

2. Power on
Press and hold the On/Off button until the display screen turns on, if it is not already turned on. The programmed number of breaths will appear with the words "Breaths left" and the battery icon at the bottom.
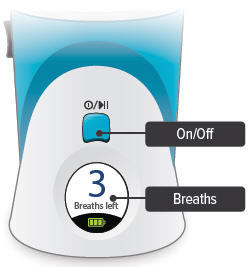
3. Adjust volume
With the Run / Program switch in the Run position, press the Volume / Breaths toggle button to access the Adjust Volume screen. Push + on the Volume / Breaths toggle button to increase beeping volume, or push - to decrease beeping volume.
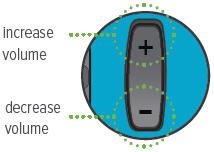

audio off mid-level volume maximum volume 4. Power off
After adjusting the beeping volume up or down, the screen will display your new setting for a couple of seconds then return to the screen displaying the breaths left.
Press and hold the On/Off button until the display screen shuts off (note: letting the button go before the screen shuts off will start a treatment session).

Preparing and using your TYVASO Inhalation System for daily treatments
Section overview
This section provides instructions for preparing and using your TYVASO Inhalation System for daily treatments.
What you will need: ▶ A clean place to take your medicine ▶ TYVASO Inhalation device ▶ TYVASO Inhalation supplies ▶ One ampule of TYVASO Inhalation Solution What is covered in this section: A: Prepare a proper environment 28 B: Gather supplies 29 C: Fill water chamber and medicine cup 32 D: Assemble inhalation device 35 E: Power on inhalation device 40 F: Inhale your medicine 42 Important:
Before using the TYVASO Inhalation System, you should:
- Wash your hands.
- Make sure the device is resting on a stable, flat surface during assembly.
A: Prepare a proper environment Follow these important instructions before setting up your treatment:
- Use the device in a quiet, distraction-free area.
- Try to use the device at times when your treatment will not be interrupted. If needed, you can pause your treatment (see page 44).
- Use the device in a comfortable space where you can stand or sit in an upright position.
- Use the device in an area where you can access a power source if you need to use the AC wall plug.
- The TYVASO Inhalation System is recommended for use indoors.
- Use the device in an area that provides enough space for the TYVASO Inhalation System and its accessories.
- Gather all necessary supplies on a stable, flat surface for assembly (see page 29 for list of supplies).
- Store the inhalation device at 15°C to 30°C (59°F to 86°F). Use at 15°C to 25°C (59°F to 77°F).
- Use the device in a well-lit area where you can clearly read these instructions, labels on the device, and the device screen.
B: Gather supplies Gather the following supplies before starting treatment. Use only the supplies provided in the starter kit and monthly refill kit for correct device function. Prior to use, inspect each part and do not use parts if they appear damaged or dirty.
- *
- These accessories are replaced every month. Replacement accessories are included in the Monthly Refill Kit.

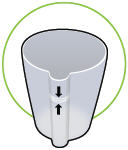
Inhalation device
Powered offTYVASO ampules
Use 1 ampule per dayWater level cup
with 45 mL of distilled water


Filter membranes*
Use 2 per dayMedicine cups*
Use 1 per dayDome assembly* 

Mouthpiece* Inhalation piece* 2 filter shells* 


2 Plugs*
(Used when storing the device)Carrying case Pen or pencil (not provided) to record your treatment 


Distilled water carrier AC wall plug Treatment Tracker Example C: Fill water chamber and medicine cup Important:
-
Wash your hands

- Unplug device when filling to avoid damage to cords or connectors.
- Only use distilled water in the device. Distilled water is highly purified water that is required for the device to function properly. If you use another type of water (such as bottled or tap water), the device might not function properly. You can purchase distilled water at most grocery stores and pharmacies.
1. Fill water chamber
Fill the water level cup with distilled water up to the arrow markers on the water cup. Use fresh distilled water each day (i.e., do not use water left in the water chamber from the previous day). Pour the distilled water into the water chamber.
Make sure the water level is above the upper, silver sensor and below the blue ring in the water chamber (about 45 mL of distilled water).
Do not overfill the water chamber, or else the medicine cup might not fit correctly.
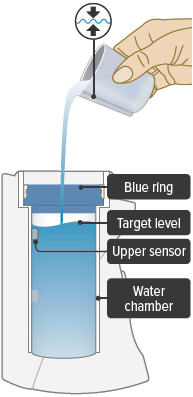
2. Place medicine cup
Obtain 1 new medicine cup and inspect it. Do not use a medicine cup that is damaged (e.g., cracked or contains holes or dents), dirty, or was used before.
Place the empty medicine cup into the water chamber of the device, making sure that the cup's bottom tip is in the distilled water.
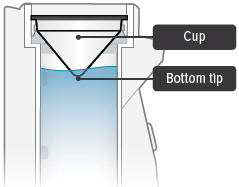
 CAUTION: Make sure you place only 1 medicine cup. Placing multiple cups will prevent the flow of medicine.
CAUTION: Make sure you place only 1 medicine cup. Placing multiple cups will prevent the flow of medicine.3. Gather one ampule
Carefully cut open the top of the foil pouch, making sure not to cut the ampules. Each pouch contains 4 ampules. Remove 1 ampule of TYVASO.
Keep unused ampules in the foil pouch because the TYVASO medicine is sensitive to light. Write the date you first opened the pouch on the foil pouch.
One ampule contains enough medicine for 1 day of treatment no matter how many breaths your doctor has prescribed.

 CAUTION: Ampules must be used within 7 days of opening foil pouch. Open only 1 pouch at a time. Throw away (discard) any unused ampules after 7 days.
CAUTION: Ampules must be used within 7 days of opening foil pouch. Open only 1 pouch at a time. Throw away (discard) any unused ampules after 7 days.4. Open ampule
Gently hold the ampule in the upright (top up) position and twist off its top.
 CAUTION: If any medicine from the ampule spills on your hands, wash your hands right away. Medicine contact with the skin can cause irritation.
CAUTION: If any medicine from the ampule spills on your hands, wash your hands right away. Medicine contact with the skin can cause irritation.5. Squeeze ampule
Point the ampule straight down toward the medicine cup's center to avoid spills.
Gently squeeze the medicine out of the ampule into the medicine cup. Squeeze until it is empty. Check to see that all of the medicine is in the medicine cup.
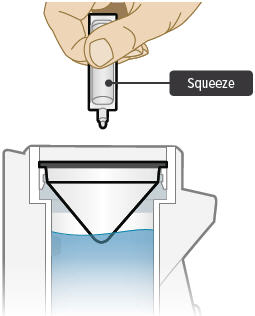
D: Assemble inhalation device Important: Do not force parts together.
The TYVASO Inhalation System is designed so the parts only fit together properly one way. When the device is assembled correctly, the parts should fit together easily.
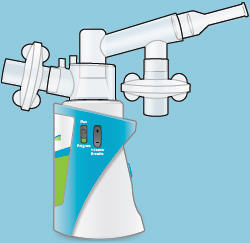
1. Check dome assembly
Visually check to make sure the black ring is securely placed in the dome assembly. The black ring should look like it does in the pictures below.
If the black ring is loose or missing, do not use the dome assembly. Throw it away and get a new one. If you need to order a new dome assembly, contact your specialty pharmacy provider.
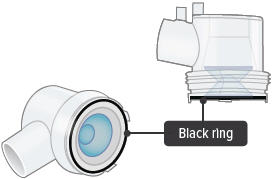
2. Attach dome assembly
Align the raised circle on the side of the dome assembly with the raised circle on the side of the device.
Push down and screw the dome assembly onto the device clockwise (right) until the filter shell port is tight and pointed to the back of the device. You will hear clicks (or a slight crunching sound) as the dome assembly presses down on the medicine cup.
Important: The dome assembly "clicks" only the first time it connects to the medicine cup. If you then realign the dome assembly you will not hear another click.
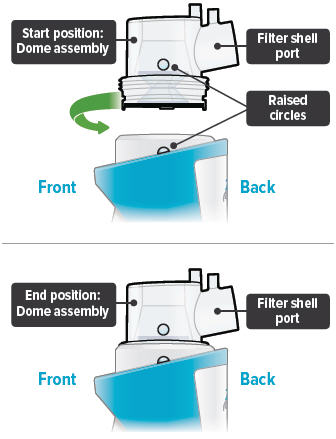
3. Install new filter membrane
Each day you will need to use a new filter membrane in each filter shell.
Note: New filter shells come with fresh filter membranes already installed.
To install a new filter membrane: ▶ a. Open the filter shell by unscrewing the 2 halves. ▶ b. Place a new filter membrane in 1 of the filter shell halves. ▶ c. Close the filter shell by screwing the 2 halves together until you can twist no further. ▶ d. Repeat steps a-c for second filter shell. 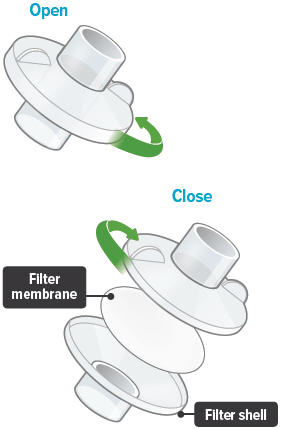
4. Attach filter shells
Insert 1 filter shell into the filter shell port on the side of the dome assembly and insert the second filter shell into the port on the bottom of the inhalation piece. The filter shells are the same and can be used in either port. You can turn the filter shells around to fit into the ports, as needed.
Make sure to insert filter shells straight into ports, not at an angle.
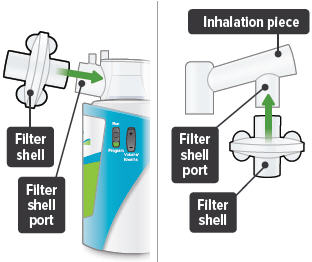
5. Insert inhalation piece
Insert the inhalation piece with attached filter shell into the upper opening of the dome assembly and turn it toward the front of the device. Gently push down the inhalation piece to make sure it is securely inserted in the dome assembly.
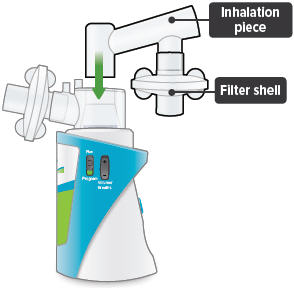
6. Insert mouthpiece
Carefully insert the mouthpiece into the inhalation piece.
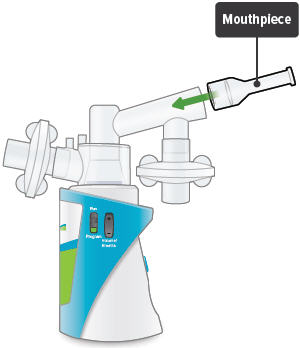
7. Check assembled device
When the device is fully assembled, it should look like it does below. Slightly turn the inhalation piece so you can see the display screen, which provides important prompts during your treatment.
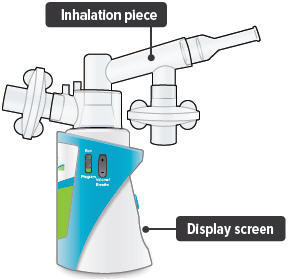
Important: Do not use device if you see liquid leaking from bottom of the device.
E: Power on inhalation device 1. Power on device
Press and hold the On/Off button until the screen turns on and the device beeps once.
The screen will display the splash screen, then the time since your last treatment, then the current breaths programmed for each dose.
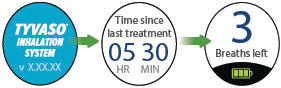
Important: Make sure the number on screen above "Breaths left" matches the prescribed number of breaths for that treatment session. If it does not match, see page 22 for instructions on setting the number of breaths for a treatment session.
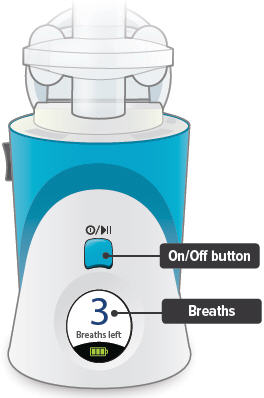
2. Plug in device, if needed
If the device's internal battery is too low to deliver a full treatment, the screen will display an instruction to plug in the power to charge the device battery. If the battery is fully depleted, the screen will not turn on.

You can charge the battery at any time, before the screen displays "Charge battery."
You can conduct a treatment session with the power plugged in. First, plug the AC wall plug's white connector into the port on the back of the inhalation device. Then, plug the AC wall plug into the wall outlet. The power status light above the port will light green when properly plugged in.
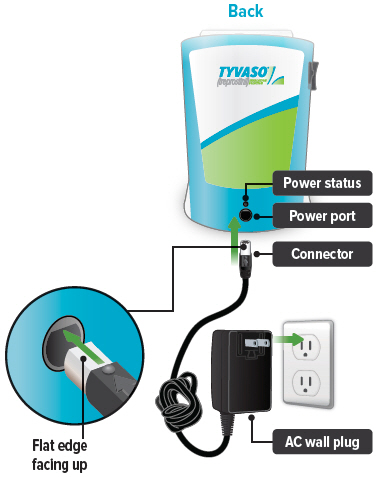
F: Inhale your medicine 1. Before starting, confirm treatment
You will breathe in (inhale) TYVASO during 4 treatment sessions each day (evenly spaced during your waking hours). During each treatment session, you will take a series of breaths through the mouthpiece of the TYVASO Inhalation System.
Before inhaling your medicine, ensure the Run/Program switch is in the 'Run' position, and make sure the number displayed on screen matches your prescribed number of breaths for that treatment session. During the treatment the device counts down each breath after a set time interval. Once you complete all breaths, record the breath number in your Treatment Tracker.
Important: If the number of breaths displayed does not match the number of breaths in your prescription, see page 22 "Setting your prescribed dose" and repeat steps 1-4.
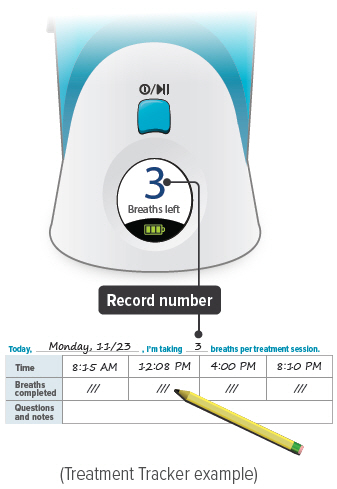
2. Hold the device upright
Hold the device upright and stand or sit in an upright position as shown below. Avoid covering the bottom of the device so that the audio speaker is not blocked.
Make sure you can see the display screen and lights clearly and that your hands do not cover the display screen or lights while holding device. If needed, you can move the inhalation piece and mouthpiece slightly to either side to see the screen and lights better.
See next page to start treatment.
Inhalation tips:
Technique:
When breathing each TYVASO treatment, be sure to keep the device level, directing the flow of medicine into the throat and not toward the roof of the mouth.
Seal your lips around the mouthpiece to ensure that you can inhale the full amount of TYVASO after it is produced by the device.
Inhalation:
Each breath should last approximately 3 seconds, breathing "normal full breaths." Do not hold your breath. Remove your lips from the mouthpiece, breathe out (exhale) normally and prepare for the next breath.
3. Press blue button to start treatment
If you need to pause treatment, you can press and immediately release (do not hold down) the blue button. Press the button again and immediately release to resume treatment. (Note: If you do not resume treatment after pausing, power off device.)

4. Wait
Look at the display screen for cues. Wait until you hear 2 short beeps. When you hear 1 long beep, exhale to prepare to inhale.

5. Inhale
When you hear 1 short beep and the indicator lights flash green, place your lips securely around the mouthpiece and inhale for 3 seconds. When lights stop flashing, remove lips from mouthpiece and exhale normally.

6. Repeat for each breath left
The screen will decrease the number of breaths left by 1. Repeat steps 4 and 5 for the number of prescribed breaths.
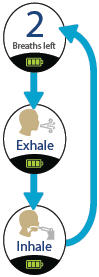
7. Finish session
After displaying the last breath sequence, the green Done screen appears, you will hear a beep, and your treatment is done.

If the device is left in the "✓ Done" mode for more than 60 seconds, it will turn off automatically.
8. Record breaths, turn off device
Record the number of breaths you inhaled on the Treatment Tracker.
Press and hold blue button until screen turns off.

 CAUTION: If medicine does not appear to be flowing properly, the system might be set up incorrectly. See "Troubleshooting", starting on page 64 for details.
CAUTION: If medicine does not appear to be flowing properly, the system might be set up incorrectly. See "Troubleshooting", starting on page 64 for details.Cleaning and Storing your TYVASO Inhalation System
Section overview
This section provides instructions for storing your TYVASO Inhalation System after each treatment and daily and weekly cleaning.
There is also information about your monthly refill kits, replacing your device, and recharging the device's battery.
What you will need: ▶ A clean place to work with the device ▶ TYVASO Inhalation device ▶ TYVASO Inhalation supplies What is covered in this section: A: Storing between sessions during the day 48 B: End of day cleaning 52 C: Recharging the battery 57 D: Weekly cleaning 59 E: Monthly Refill Kit 60 F: Replacing your devices 61 Important:
For further support, you can:
- Fill out and refer to your emergency contact information on the back of this Instructions for Use manual.
- Call 1-877-UNITHER (1-877-864-8437) for questions and information, or to report an adverse reaction.
A: Storing between sessions during the day If you have more treatment sessions left in the day, perform the steps in this section.
If you have completed your last treatment session of the day, skip to "End of day cleaning" on page 52.
Be sure to pack all parts, including the AC wall plug, in the carrying case whenever transporting your device.
1. Disconnect AC wall plug
(if it is currently connected)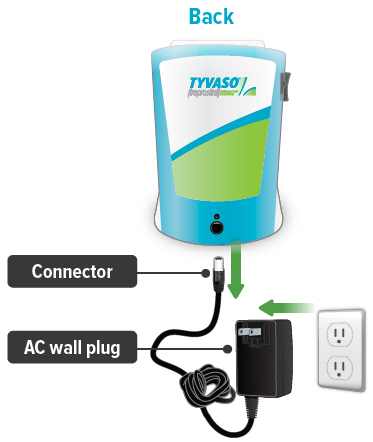
2. Remove mouthpiece
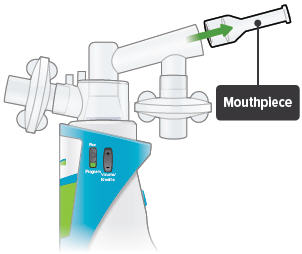
3. Remove inhalation piece
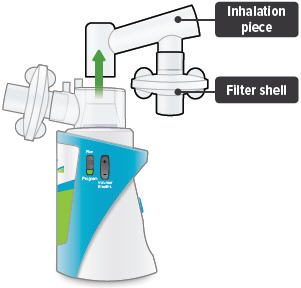
Important: When removing accessories between treatment sessions, hold the device by its base to avoid spilling the medicine.
4. Remove both filter shells
Note: Do not remove the filter membranes from filter shells until after the last treatment session of the day.
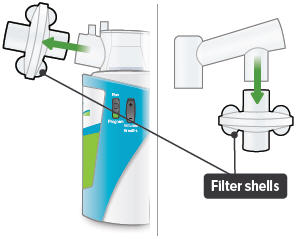
5. Leave dome assembly
Leave dome assembly and medicine cup (with the medicine still in it) attached to the device.
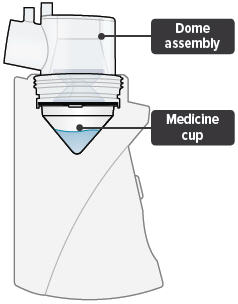
6. Place plugs in dome assembly
Insert a plug into each of the 2 open holes on the dome assembly to prevent the medicine from spilling out.
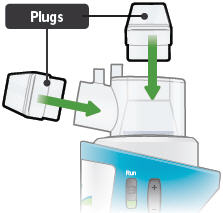
Important: If the plugs are not in place, the medicine may spill. If you spill any medicine, discard remaining medicine and start your next treatment with a new ampule.
7. Store in carrying case
You can store the inhalation device with the plugged dome assembly and disassembled accessories in the carrying case between treatment sessions.
Keep the carrying case upright while inserting the device and components so that water and medicine does not spill out of the device.
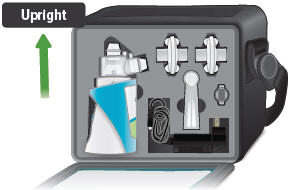
Important: Store the inhalation device in an upright position until the next treatment session. See "Specifications" on page 76 for additional storage and transport information.
B: End of day cleaning If you have completed your last treatment session of the day, perform the steps in this section.
If you have more treatment sessions left in the day, refer back to "Storing between sessions during the day" on page 48.
1. Disconnect AC wall plug
(if it is currently connected)
2. Remove mouthpiece
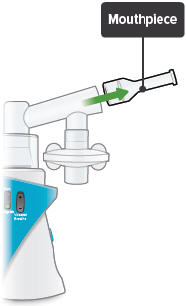
3. Remove inhalation piece with attached filter shell

4. Remove both filter shells
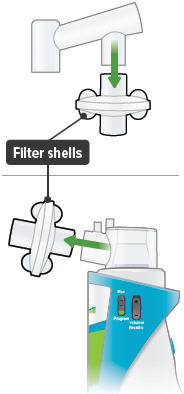
5. Discard filter membranes
Open filter shells by twisting in opposite directions. Remove and discard used filter membranes in the trash.
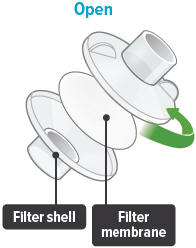
6. Remove dome assembly
Remove the dome assembly by turning it counter-clockwise (to the left). The medicine cup should stay attached to the dome assembly.
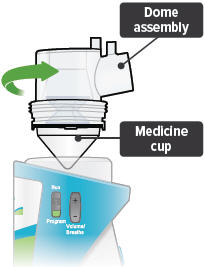
7. Remove medicine cup
Remove the medicine cup by gently squeezing on the sides where it is attached to the dome assembly.
Be careful not to spill any leftover medicine.
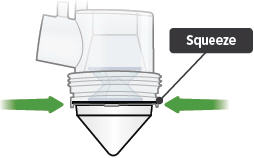
 CAUTION: If any medicine from the medicine cup spills on your hands, wash your hands immediately. Medicine contact with the skin can cause irritation.
CAUTION: If any medicine from the medicine cup spills on your hands, wash your hands immediately. Medicine contact with the skin can cause irritation.8. Empty medicine cup
Empty any leftover medicine in the medicine cup into a waste basket, and discard the medicine cup.
Important: Discard remaining TYVASO® (treprostinil) Inhalation Solution in an appropriate waste receptacle. Discard plastic medicine cup in the trash.
Do not reuse or recycle medicine cup.
9. Empty and clean device
Empty distilled water from water chamber and let inhalation device air dry upside down. You can wipe the water chamber with a soft cloth or paper towel to remove any remaining water.
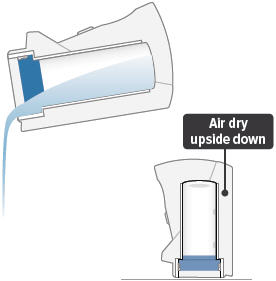
Important: Do not place the inhalation device in water or in a dishwasher.
10. Clean accessories
Clean accessories (pictured below) by hand in mild, soapy, warm water, then rinse them thoroughly with water. Allow accessories to air dry.

Dome assembly Inhalation piece 

Mouthpiece Filter shells Important: Do not place the inhalation device or its accessories in a microwave, conventional oven, or dishwasher.
11. Store components
Once all the items are dry, you can store the filter shells, inhalation piece, mouthpiece, dome assembly, AC wall plug, and inhalation device in the carrying case until the next day's treatment sessions.
You can also recharge the device for the next day of use (see page 57).

C: Recharging the battery 1. Checking the battery's status
You can recharge your battery at any time. Press and hold the blue button to power on the device to check battery status. Make sure the Run / Program switch is set to Run.
- The battery icon at the bottom of the screen indicates battery status:
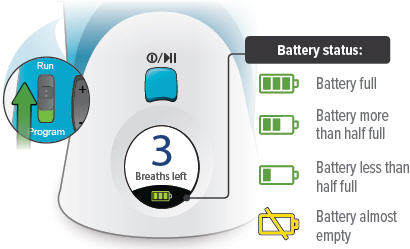
- "Charge battery" appears on screen if there is not enough charge to conduct a treatment session.

Important: Always charge the device before you first use it. You should also charge the device when not in use and in between uses.
2. Charging the battery
Plug the AC wall plug's white connector into the port on the back of the inhalation device. Then, plug the AC wall plug into the wall outlet. The power status light above the port will light green when properly plugged in.
The device battery might take up to 8 hours to fully charge.
If the device is powered on, the battery charging icon appears next to the battery icon at the bottom of the screen.

Battery charging icon 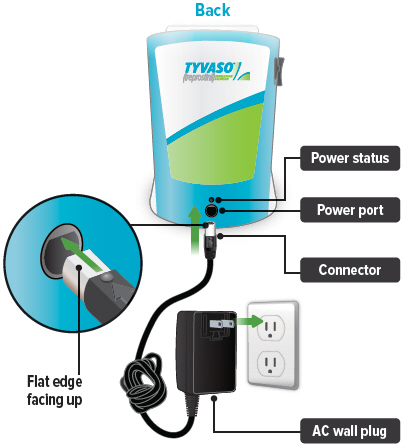
D: Weekly cleaning Clean the device once a week to help avoid corrosion and leaks and to keep your device working properly.
Once a week, use a clean, dry cloth to wipe the interior of the water chamber. Make sure to wipe the 2 silver sensors and the white disc in the bottom of the water chamber.
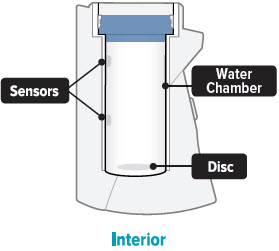
You may wipe the exterior of the device with a damp cloth if the lights or buttons become difficult to see.

E: Monthly Refill Kit Once a month, you will receive a refill kit that will come with a new set of accessories from your specialty pharmacy provider.
- Inspect the shipment to be sure all parts are included.
- Once the new kit has arrived, discard the used dome assembly, inhalation piece, mouthpiece, filter shells, and plugs.
- Do not recycle the used accessories.

Dome assembly 2 filter shells 


Mouthpiece Inhalation piece 2 plugs 

Filter membranes Medicine cups F: Replacing your devices The inhalation devices should be replaced every 2 years from your first day of use. Replacement inhalation devices will be supplied by your specialty pharmacy provider.
When you receive a new inhalation device your specialty pharmacy provider will provide instructions for returning the old device.
Help / More information about your TYVASO Inhalation System
Section overview
This section provides additional information about your device. Use this section to troubleshoot difficulties you have with the device, or to learn more about the device's specifications and warranty.
What you will need: ▶ Access to a phone (to contact support if troubleshooting steps do not resolve the problem) ▶ A clean place to work with the device ▶ TYVASO Inhalation Device or supplies, as needed What is covered in this section: A: Troubleshooting 64 B: Specifications 76 C: Electromagnetic compatibility (EMC) 79 D: Glossary 87 E: Warranty information 90 Important:
For further support, you can:
- Fill out and refer to your emergency contact information on the back of this Instructions for Use manual.
- Call 1-877-UNITHER (1-877-864-8437) for questions and information, or to report an adverse reaction.
A: Troubleshooting Problem Possible causes Corrective actions Charge Battery screen appears

Low battery Charge the device battery by attaching the AC wall plug to an outlet. You can conduct a treatment session with the device plugged in. AC wall plug not properly connected

Ensure that the plug adapter piece (the detachable piece with the metal prongs) is securely attached to the AC wall plug. Then, make sure the AC wall plug is properly connected to an outlet and device. The status lights on the back of the AC wall plug and device should light green. You can conduct a treatment session with the device plugged in. AC wall plug is defective

Use the replacement AC wall plug. Confirm that status light on AC wall plug is green when plugged in. You can conduct a treatment session with the device plugged in. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. Screen does not turn on

Device battery is completely empty Charge the device battery by attaching the AC wall plug to an outlet. You can conduct a treatment session with the device plugged in. AC wall plug not properly connected

Ensure that the plug adapter piece (the detachable piece with the metal prongs) is securely attached to the AC wall plug. Then, make sure the AC wall plug is properly connected to an outlet and device. The status lights on the back of the AC wall plug and device should light green. You can conduct a treatment session with the device plugged in. AC wall plug is defective

Use the replacement AC wall plug. Confirm that status light on AC wall plug is green when plugged in. You can conduct a treatment session with the device plugged in. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. Call Support screen appears

Temporary device failure. If device is plugged in, unplug device and wait for the device to power off. Power on device from internal battery and check that Call Support screen does not reappear. Continue taking breaths to complete the treatment session. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. Loss of power during treatment Device is disconnected from power source and battery is empty Reconnect device to power source and confirm the power status light on back of device is green (battery is charging). Press and hold the blue On/Off button to turn on the device. The display will show how many breaths are left in that treatment session. Press and immediately release (do not hold down) the blue button again to continue your treatment session. Power source is temporarily disrupted (for example, electricity interruption due to a storm) Reconnect device to power source and confirm the power status light on back of device is green (battery is charging). Press and hold the blue On/Off button to turn on the device. The display will show how many breaths are left in that treatment session. Press and immediately release (do not hold down) the blue button again to continue your treatment session. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. Add Water screen appears

Water chamber is empty or distilled water level is too low.
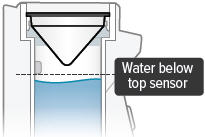
Unplug device, if plugged in, and power off device. Remove dome assembly (making sure not to spill medicine) and place it aside, keeping it upright. Then empty water chamber.
Refill water chamber with distilled water using water level cup (see page 32). Reassemble device. Power the device on and continue treatment.The distilled water is too pure. Unplug device, if plugged in, and power off device. Remove dome assembly (making sure not to spill medicine) and place it aside, keeping it upright. Then empty water chamber.
Add 1 teaspoon of tap water to the water level cup. Fill rest of cup with distilled water up to level between the 2 arrow markings on cup (see page 32). Pour cup's contents into water chamber. Reassemble device. Power the device on and continue treatment.Water level sensors have a thin layer of build-up
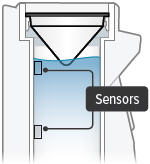
Unplug device, if plugged in, and power off device. Remove dome assembly (making sure not to spill medicine) and place it aside, keeping it upright. Then empty water chamber.
Clean sensors and interior surfaces of water chamber with a clean cloth. Refill water chamber with distilled water using water level cup (see page 32). Reassemble device. Power the device on and continue treatment.If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. No "click" (or crunch) was heard when attaching the dome assembly No medicine cup in the water chamber of the device Unplug device, if plugged in, and power off device. Place an empty medicine cup into the water chamber of the device and fill it with 1 ampule of TYVASO. Reassemble device. Power the device on and continue treatment. Multiple medicine cups attached to the dome assembly
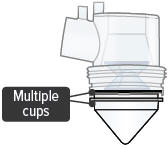
Unplug device, if plugged in, and power off device. Remove and dispose of all medicine cups in the device. Place a single, new medicine cup into device water chamber and fill with 1 ampule of TYVASO. Reassemble device. Power the device on and continue treatment. Dome assembly is not securely in place Unplug device, if plugged in, and power off device.
Align the raised circle on the side of the dome assembly with the raised circle on the side of the device.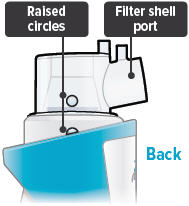
Push down and screw the dome assembly onto the device clockwise (right) until the filter shell port is tight and pointed to the back of the device and the raised circles line up again. You will hear clicks (or crunch sound) as the dome assembly presses down on the medicine cup. Reassemble device. Power the device on and continue treatment. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. No medicine comes out of the device during a treatment session No TYVASO® (treprostinil) Inhalation Solution in the medicine cup
Unplug device, if plugged in, and power off device. Fill medicine cup with 1 ampule of TYVASO. Reassemble device. Power the device on and continue treatment. Damaged medicine cup

Unplug device, if plugged in, and power off device. Remove and dispose of the medicine cup in the device. Empty the water chamber then refill it with 45 mL of distilled water (see page 32). Place a single, new medicine cup into water chamber and fill with 1 ampule of TYVASO. Reassemble device. Power the device on and continue treatment. Distilled water level in the water chamber is too high
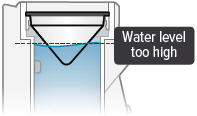
Unplug device, if plugged in, and power off device. Remove dome assembly (making sure not to spill medicine) and place it aside, keeping it upright. Empty the water chamber then refill it with 45 mL of distilled water (see page 32). Reassemble device. Power the device on and continue treatment. Multiple medicine cups attached to the dome assembly
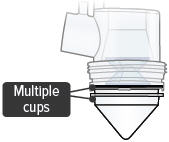
Unplug device, if plugged in, and power off device. Remove and dispose of all medicine cups in the device. Place a single, new medicine cup into water chamber and fill with 1 ampule of TYVASO. Reassemble device. Power the device on and continue treatment. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. Difficult to breathe in medicine through the mouthpiece Filter membrane is clogged
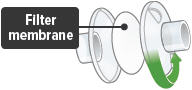
Unplug device, if plugged in, and power off device. Replace both filter membranes (see page 37). Reassemble device. Power the device on and continue treatment. If device still does not function after taking corrective actions listed above, contact your specialty pharmacy provider for assistance. B: Specifications Inhalation Device Model TD-300/A Size 3.5" × 3.2" × 4.7" (90 × 82 × 120 mm) Weight, inhalation device 365 g (12.8 oz) Types of power supply AC wall plug, 120 V, 60 Hz Power input 14 V DC, 1.1 A maximum Operating power consumption 18 Watt maximum Ultrasonic frequency 2.4 MHz (nominal) Nebulization rate 0.50 - 0.55 mg/min (0.9% Saline) Medicine cup capacity 6 mL, nominal Water chamber capacity 45 mL, nominal Electric protection class II, Type BF Storage temperature/humidity 15 to 30ºC/20-80% relative humidity Operating temperature/humidity 15 to 25ºC/40-75% relative humidity A-weighted sound pressure level 75 dBA (1 m), maximum Packaging Dimensions (Approximate Length × Width × Height) Patient Starter Kit (PSK) 12.2" × 14.3" × 16.0" Monthly Refill Kit (MRK) 9.9" × 6.1" × 16.1" Institutional Starter Kit (ISK) 12.2" × 14.3" × 16.0" TYVASO Mass and Particle Specifications for 9 breaths Mass Median Aerodynamic Diameter (MMAD)* mean = 2.0 µm
SD = 0.3Total Emitted Dose per Breath† mean = 6.0 µg
SD = 0.4Total Aerosol Mass* mean = 58 µg
SD = 5.9Total Respirable Dose* mean = 44.6 µg
SD = 3.5Respirable Fraction* mean = 73%
SD = 5Geometric Standard Deviation (GSD)* mean = 2.6
SD = 0.4Accessories Note: Part number subject to change. TD-300N-US AC wall plug ON-102/1/C Medicine cup, Quantity-16 ON-109 Filter membranes ON-120/C Plugs ON-101/C Filter shell TD-103/C Dome assembly with baffle plate ON-104/C Inhalation piece ON-105/C Mouthpiece TD-118 Water level cup TD-158 Carrying case TD-155 Distilled water carrier C: Electromagnetic compatibility (EMC) The TYVASO Inhalation System has been tested and found to comply with the electromagnetic compatibility (EMC) limits for medical devices according to IEC 60601-1-2: (2007). Compliance is intended to provide reasonable protection against harmful interference in a typical user environment.
Table 1, Table 2 and Table 3 document the intended EMC use environment and established compliance levels for the TYVASO Inhalation System. To ensure the intended performance, use the system in the environments described in these tables.
The TYVASO Inhalation System is intended for use in the electromagnetic environment specified in this section.
Table 1: Guidance and manufacturer's declaration - electromagnetic emissions Guidance and manufacturer's declaration - electromagnetic emissions The TYVASO Inhalation System is intended for use in the electromagnetic environment specified below. The customer or the user of the TYVASO Inhalation System should assure that it is used in such an environment. Emissions test Compliance Electromagnetic environment - guidance RF emissions CISPR 11 Group 1 The TYVASO Inhalation System uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF emissions CISPR 11 Class B The TYVASO Inhalation System is suitable for use in all establishments, including domestic establishments and those directly connected to the public low voltage power supply network that supplies buildings used for domestic purposes. Harmonic emissions IEC 61000-3-2 Class A Voltage fluctuations/flicker emissions IEC 61000-3-3 Complies Table 2: Guidance and manufacturer's declaration – electromagnetic immunity NOTE: UT is the a.c. mains voltage prior to application of the test level.
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.- *
- Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the TYVASO Inhalation System is used exceeds the applicable RF compliance level above, the TYVASO Inhalation System should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the TYVASO Inhalation System.
- †
- Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m.
Guidance and manufacturer's declaration – electromagnetic immunity The TYVASO Inhalation System is intended for use in the electromagnetic environment specified below. The customer or the user of the TYVASO Inhalation System should assure that it is used in such an environment. Immunity test IEC 60601 Test level Compliance level Electromagnetic environment - guidance Electrostatic discharge (ESD)
IEC 61000-4-2± 8 kV contact
± 15 kV air± 8 kV contact
± 15 kV airFloors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical fast transient/burst
IEC 61000-4-4± 2 kV for power supply lines
± 1 kV for input/output lines± 2 kV for power supply lines
± 1 kV for input/output linesMains power quality should be that of a typical commercial or hospital environment. Surge
IEC 61000-4-5± 1 kV line(s) to line(s)
± 2 kV line(s) to earth± 1 kV line(s) to line(s)
± 2 kV line(s) to earthMains power quality should be that of a typical commercial or hospital environment. Voltage dips, short interruptions and voltage variations on power supply input lines
IEC 61000-4-110 % UT (100 % dip in UT) for 0,5 cycle
0 % UT (100 % dip in UT) for 1 cycle
70 % UT (30 % dip in UT) for 25/30 cycles
0 % UT (100 % dip in UT) for 250/300 cycle0 % UT (100 % dip in UT) for 0,5 cycle
0 % UT (100 % dip in UT) for 1 cycle
70 % UT (30 % dip in UT) for 25/30 cycles
0 % UT (100 % dip in UT) for 250/300 cycleMains power quality should be that of a typical commercial or hospital environment. If the user of the TYVASO Inhalation System requires continued operation during power mains interruptions, it is recommended that the TYVASO Inhalation System be powered from an uninterruptible power supply or the internal battery. Power frequency (50/60 Hz) magnetic field
IEC 61000-4-830 A/m 30 A/m Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-33 Vrms 150 kHz to 80 MHz
10 V/m 80 MHz to 2.6 GHz3 Vrms 150 kHz to 80 MHz
10 V/m 80 MHz to 2.6 GHzPortable and mobile RF communications equipment should be used no closer to any part of TYVASO Inhalation System, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance
d = 1.2 √P
d = 1.2 √P 80 MHz to 800 MHz
d = 2.3 √P 800 MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey*, should be less than the compliance level in each frequency range†.
Interference may occur in the vicinity of equipment marked with the following symbol:
Table 3: Manufacturer's Declaration – Recommended separation distances between portable and mobile communications equipment and the TYVASO Inhalation System For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.Recommended separation distances between portable and mobile RF communications equipment and the TYVASO Inhalation System The TYVASO Inhalation System is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the TYVASO Inhalation System can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the TYVASO Inhalation System as recommended below, according to the maximum output power of the communications equipment. Rated maximum output power of transmitter
WSeparation distance according to frequency of transmitter
m150 kHz to 80 MHz
d = 1.2 √P80 MHz to 800 MHz
d = 1.2 √P800 MHz to 2.5 GHz
d = 2.3 √P0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 D: Glossary Accessories: Parts of the TYVASO Inhalation System. See page 29.
Ampule: A sealed, lightweight clear plastic vial containing a 1-day supply of TYVASO® (treprostinil) Inhalation Solution.
Black ring: A round seal that fits on the bottom of the dome assembly. The seal helps ensure that TYVASO does not mix with the distilled water in the device water chamber.
Display screen: A small area on the inhalation device that provides instructions and device information.
Distilled water: Water that is highly purified so that it contains only essential elements.
Dome assembly: The plastic accessory that contains the baffle plate and connects the mouthpiece, inhalation piece, and filter shells to the base of the inhalation device.
Filter membrane: The white pad that goes into the filter shells.
Filter shells: Plastic accessories that hold the filter membranes.
Inhalation indicator lights: Two green lights on the top surface of the inhalation device that signals when you should inhale.
Inhalation piece: The plastic accessory that connects the mouthpiece with the dome assembly.
Inhalation device: The base of the TYVASO Inhalation System to which the accessories connect. The inhalation device contains the display screen and lights.
Inhale: How you will breathe in TYVASO with the TYVASO Inhalation System.
Medicine cup: The disposable plastic cone-shaped cup into which TYVASO is poured. The medicine cup fits inside the water chamber.
Mouthpiece: The plastic part that you will breathe through (using your mouth) to inhale TYVASO.
On/Off, Start/Pause (blue) button: A manually activated control on the front of the device that switches between fully on and fully off power states. Once the device power is on, the button begins or pauses treatment.
Plugs: Plastic accessories that are inserted into the openings of the dome assembly between treatment sessions. Plugs help keep TYVASO from spilling if the inhalation device tips over.
Power status light: LED on the back of the device that lights green when power is connected and battery is charging.
Power port: Port on back of device for plugging into a power source using the AC wall plug.
Prompts: The audio and visual signals that help guide you through the treatment sessions.
Run / Program switch: A manually activated control on the side of the device that switches between the modes for delivering treatment (Run) and programming breaths (Program).
Sensors: The silver objects on the inside wall of the water chamber. The sensors must be covered with distilled water for the TYVASO Inhalation System to function properly.
Specialty pharmacy provider: A pharmacy that carries only specialized medicines and medical devices. Your specialty pharmacy provider is a good source of information about TYVASO and the TYVASO Inhalation System.
Treatment session: 1 of 4 daily sessions during which you will take TYVASO with a specific number of inhalations.
TYVASO: The prescription medicine that you will use with the TYVASO Inhalation System.
Volume / Breaths toggle button: A manually activated control on the side of the device that increases or decreases audio volume (when in Run mode) and programmed breaths (when in Program mode).
Water chamber: The white hollow portion in the center of the inhalation device into which distilled water and the medicine cup are placed.
E: Warranty information Your TYVASO Inhalation System is granted a replacement or repair warranty good for 2 years from your date of receipt of the TYVASO Inhalation System or 5 years from the date of manufacture, whichever comes first. This warranty applies to the TYVASO Inhalation System device only. Accessory components are not covered under warranty.
Circumstances that may void your warranty include: ▶ Modification or disassembly of the TYVASO Inhalation System device by anyone other than a factory-authorized technician ▶ Failure to comply with this written Instructions for Use manual when operating the TYVASO Inhalation System ▶ Unapproved use of the TYVASO Inhalation System For all inquiries relating to service or warranty for your TYVASO Inhalation System, contact your specialty pharmacy provider.
You should have the following information available: ▶ Device serial number (located on bottom of TYVASO Inhalation System) ▶ Date TYVASO Inhalation System was acquired ▶ Nature of the problem and any steps taken to fix it TYVASO ®
INHALATION SYSTEMTYVASO®
(treprostinil)
INHALATION
SOLUTIONTYVASO Inhalation Solution is for prescription use only.
Emergency contact information ▶ Clinician: ▶ Nurse educator: ▶ Specialty pharmacist: ▶ United Therapeutics: For further questions and information, or to report a problem with your device or an adverse event with your TYVASO Inhalation System, please call 1-877-UNITHER (1-877-864-8437).
Distributed by:
United Therapeutics Corporation
Research Triangle Park, North Carolina 27709© 2022, United Therapeutics Corporation.
All rights reserved.102040
TYVASO is a registered trademark of
United Therapeutics Corporation.This Instructions for Use has been
approved by the U.S. Food and Drug
Administration.Revised: August 2022
- PRINCIPAL DISPLAY PANEL - 4 Ampule Pouch Carton
- PRINCIPAL DISPLAY PANEL - 28 Ampule Pouch Carton
-
PRINCIPAL DISPLAY PANEL - Patient Starter Kit
TYVASO Inhalation System
Patient Starter Kit
TYVASO®
(treprostinil)
INHALATION
SOLUTIONTreprostinil 1.74 mg/2.9 mL (0.6 mg/mL)
TYVASO is for oral inhalation use only and is intended for administration with the TYVASO Inhalation System.
See package insert and Instructions for Use manual for dosage and administration.CONTENTS QTY CONTENTS QTY - +
- 28 ampule carton of TYVASO
(seven foil pouches, each containing four 2.9 mL ampules)
1 - +
- AC wall plugs
2 - +
- TYVASO Inhalation Device
2 - +
- Water level cup
1 - +
- TYVASO Inhalation System Instructions for Use manual
1 - +
- Plugs for storage between treatment sessions
2 - +
- Treatment Tracker
1 - +
- Carrying case
1 - +
- Sets of Dome assembly, Inhalation piece,
Mouthpiece, and 2 Filter shells
2 - +
- Distilled water carrier
1 - +
- Medicine cups
32 - +
- Filter membranes
65 Each ampule of TYVASO contains: treprostinil 1.74 mg. Each ampule also contains 18.9 mg sodium chloride,
18.3 mg sodium citrate dihydrate, 0.6 mg sodium hydroxide, 11.7 mg 1N hydrochloric acid, and water for injection.
Hydrochloric acid and sodium hydroxide may have been added to adjust pH.- +
- One new ampule should be used each day. Each ampule of TYVASO (once opened and transferred to the
medicine cup) should remain in the device for no more than 1 day. Any remaining TYVASO and the medicine
cup should be discarded at the end of each day. - +
- Once a foil pouch is opened, use ampules within 7 days.
- +
- Store unused ampules of TYVASO in the foil pouch until use, protected from light.
- +
- Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room
Temperature].
Patient Starter Kit For Single Patient Use Only.
Keep out of the reach of children.
NDC 66302-206-01 Rx onlyManufactured by United Therapeutics Corporation
Research Triangle Park, NC 27709EXP YYYY - MMM
LOT XXXXXXX
100957 Rev. 04/2021United
Therapeutics
CORPORATION
-
PRINCIPAL DISPLAY PANEL - Refill Kit
TYVASO®
(treprostinil)
INHALATION
SOLUTIONTreprostinil 1.74 mg/2.9 mL (0.6 mg/mL)
TYVASO is for oral inhalation use only and is intended for administration with the TYVASO Inhalation System.
See package insert and Instructions for Use manual for dosage and administration.
TYVASO Inhalation System Refill Kit
CONTENTS QTY - 28 ampule carton of TYVASO (seven foil pouches each containing
four 2.9 mL ampules)
1 - Set of Dome assembly, Inhalation piece, Mouthpiece, and
2 Filter shells
1 - Medicine cups
32 - Filter membranes
65 - Plugs for storage between treatment sessions
2 - Treatment Tracker
1 Each ampule of TYVASO contains: treprostinil 1.74 mg. Each ampule also contains 18.9 mg sodium chloride,
18.3 mg sodium citrate dihydrate, 0.6 mg sodium hydroxide, 11.7 mg 1N hydrochloric acid, and water for
injection. Hydrochloric acid and sodium hydroxide may have been added to adjust pH.- —
- One new ampule should be used each day. Each ampule of TYVASO (once opened and transferred to
the medicine cup) should remain in the device for no more than 1 day. Any remaining TYVASO and the
medicine cup should be discarded at the end of each day. - —
- Once a foil pouch is opened, use ampules within 7 days.
- —
- Store unused ampules of TYVASO in the foil pouch until use, protected from light.
- —
- Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)
[see USP Controlled Room Temperature].
Keep out of the reach of children.
NDC 66302-206-02
Rx onlyManufactured for United Therapeutics Corporation
Research Triangle Park, NC 27709United
Therapeutics
CORPORATIONEXP YYYY - MMM
LOT XXXXXXX
RTP3088 Rev. 04/2021
- 28 ampule carton of TYVASO (seven foil pouches each containing
-
PRINCIPAL DISPLAY PANEL - Institutional Starter Kit
TYVASO Inhalation System
Institutional Starter KitTYVASO®
(treprostinil)
INHALATION
SOLUTIONTreprostinil 1.74 mg/2.9 mL (0.6 mg/mL)
TYVASO is for oral inhalation use only and is intended for administration with the TYVASO Inhalation System.
See package insert and Instructions for Use manual for dosage and administration.CONTENTS QTY CONTENTS QTY - +
- 4 ampule carton of TYVASO
(one foil pouch containing four 2.9 mL ampules)
1 - +
- AC wall plugs
2 - +
- TYVASO Inhalation Device
2 - +
- Water level cup
1 - +
- TYVASO Inhalation System Instructions for Use manual
1 - +
- Plugs for storage between treatment sessions
2 - +
- Treatment Tracker
1 - +
- Carrying case
1 - +
- Sets of Dome assembly, Inhalation piece,
Mouthpiece, and 2 Filter shells
2 - +
- Distilled water carrier
1 - +
- Medicine cups
32 - +
- Filter membranes
65 Each ampule of TYVASO contains: treprostinil 1.74 mg. Each ampule also contains 18.9 mg sodium chloride,
18.3 mg sodium citrate dihydrate, 0.6 mg sodium hydroxide, 11.7 mg 1N hydrochloric acid, and water for injection.
Hydrochloric acid and sodium hydroxide may have been added to adjust pH.- +
- One new ampule should be used each day. Each ampule of TYVASO (once opened and transferred to the
medicine cup) should remain in the device for no more than 1 day. Any remaining TYVASO and the medicine
cup should be discarded at the end of each day. - +
- Once a foil pouch is opened, use ampules within 7 days.
- +
- Store unused ampules of TYVASO in the foil pouch until use, protected from light.
- +
- Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room
Temperature].
Institutional Starter Kit For Single Patient Use Only.
Keep out of the reach of children.
NDC 66302-206-04 Rx onlyManufactured by United Therapeutics Corporation
Research Triangle Park, NC 27709EXP YYYY-MMM
LOT XXXXXXX
100958 Rev. 04/2021United
Therapeutics
CORPORATION
-
INGREDIENTS AND APPEARANCE
TYVASO
treprostinil inhalantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-206 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 1.74 mg in 2.9 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) 18.9 mg in 2.9 mL trisodium citrate dihydrate (UNII: B22547B95K) 18.3 mg in 2.9 mL sodium hydroxide (UNII: 55X04QC32I) 0.6 mg in 2.9 mL hydrochloric acid (UNII: QTT17582CB) 11.7 mg in 2.9 mL water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-206-01 1 in 1 KIT 08/14/2009 1 28 in 1 BOX 1 2.9 mL in 1 AMPULE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:66302-206-02 1 in 1 KIT 08/14/2009 2 28 in 1 BOX 2 2.9 mL in 1 AMPULE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC:66302-206-03 4 in 1 BOX 08/14/2009 3 2.9 mL in 1 AMPULE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 4 NDC:66302-206-04 1 in 1 KIT 08/14/2009 4 4 in 1 BOX 4 2.9 mL in 1 AMPULE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022387 08/14/2009 Labeler - United Therapeutics Corporation (965460025) Establishment Name Address ID/FEI Business Operations United Therapeutics Corporation 015718364 MANUFACTURE(66302-206) , LABEL(66302-206) , PACK(66302-206) Establishment Name Address ID/FEI Business Operations United Therapeutics Corporation 965460025 MANUFACTURE(66302-206) , LABEL(66302-206) , ANALYSIS(66302-206) , API MANUFACTURE(66302-206)







