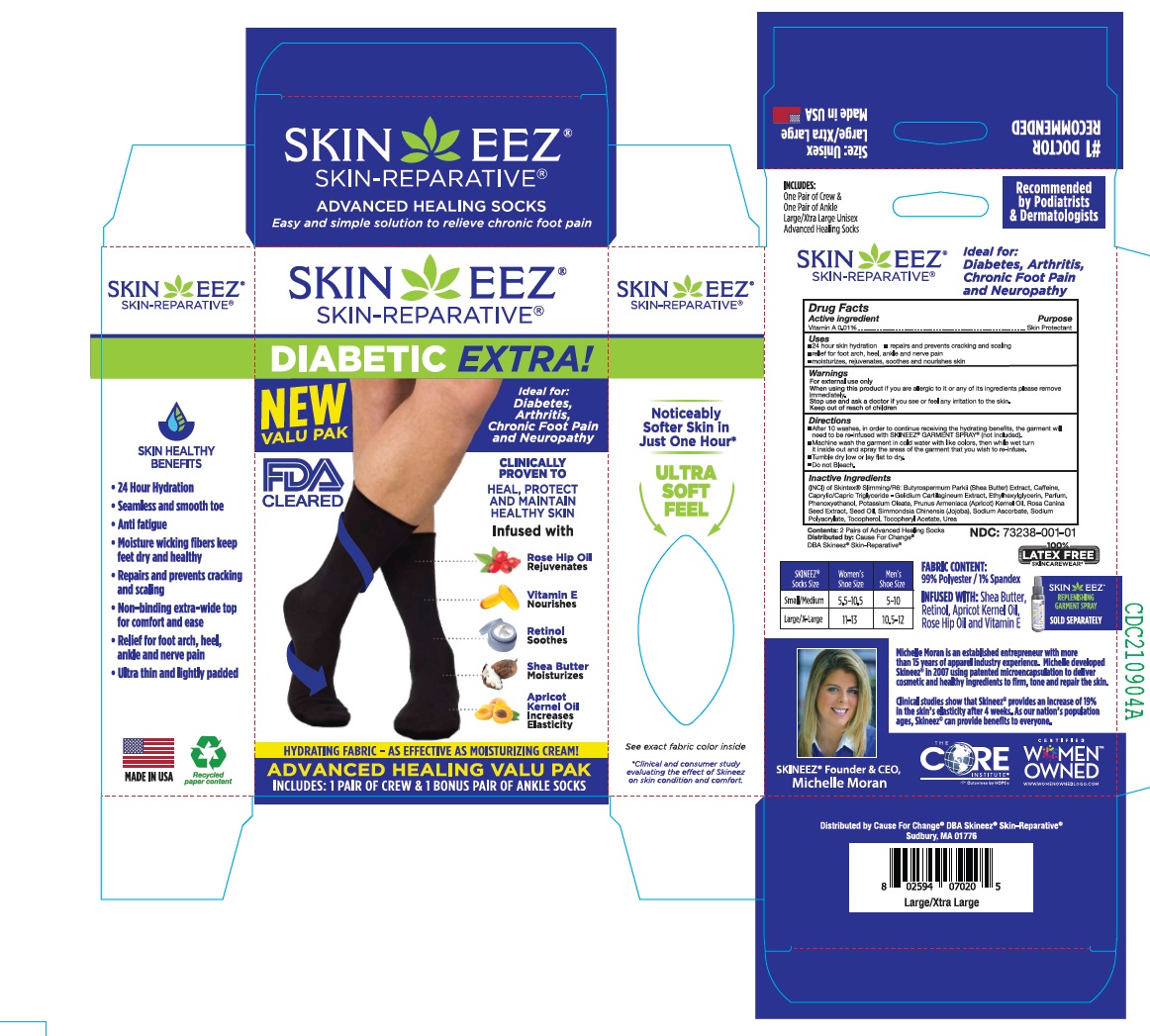
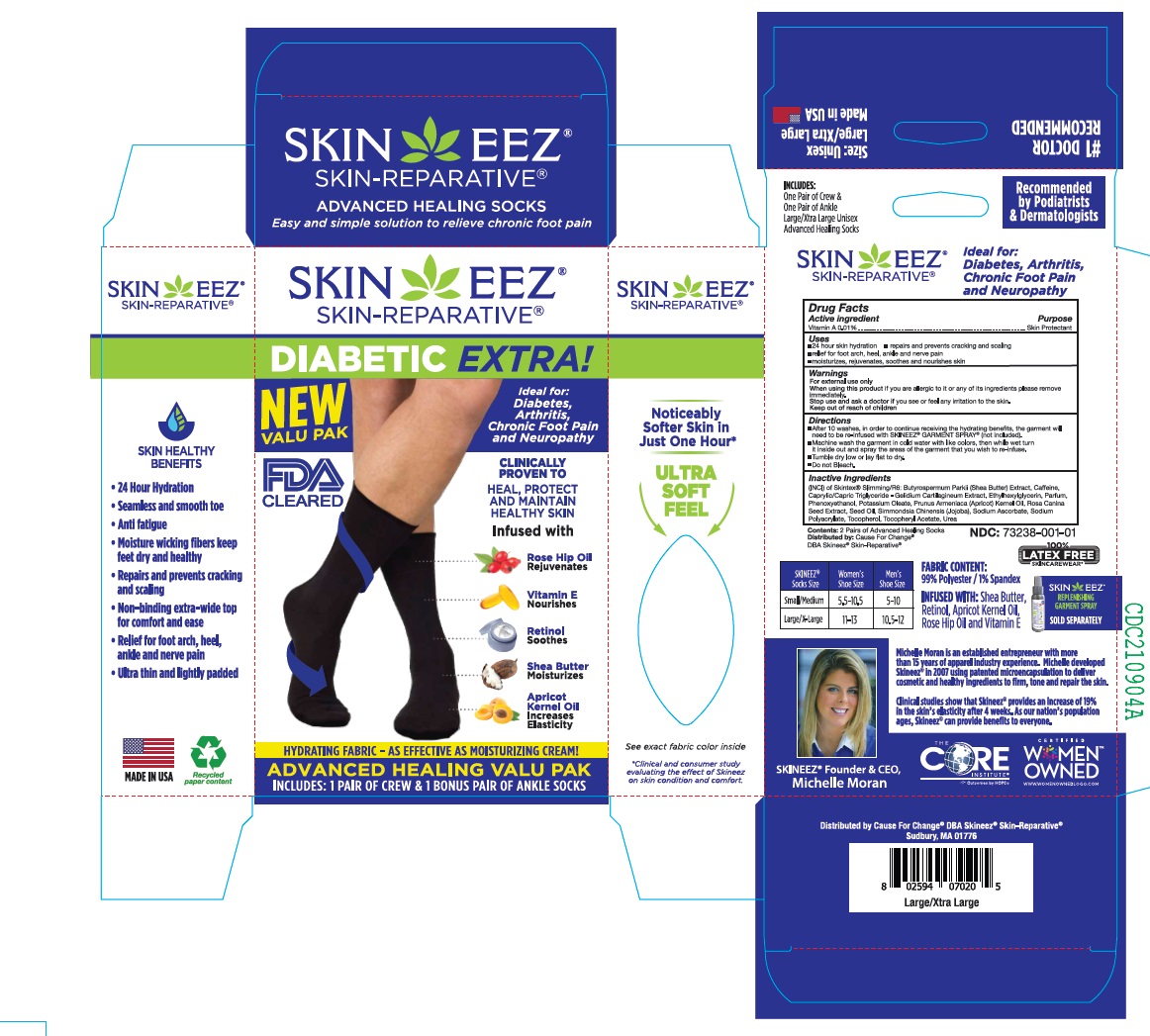
Label: SKINEEZ- vitamin a cloth
- NDC Code(s): 73238-001-01
- Packager: CAUSE FOR CHANGE LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient Purpose
- Uses
- Warnings
- Keep out of reach of children
-
Directions
• After 10 washes, in order to continue receiving the hydrating benefits, the garment will need to be re-infused with SKINEEZ® GARMENT SPRAY® (not included).
• Machine wash the garment in cold water with like colors, then while wet turn it inside out and spray the areas of the garment that you wish to re-infuse.
• Tumble dry low or lay flat to dry.
• Do not Bleach. - INDICATIONS & USAGE
-
Inactive ingredients
(INCI) of Skintex® Slimming/R6: Butyrospermum Parkii (Shea Butter) Extract, Caffeine, Caprylic/Capric Triglyceride - Gelidium Cartilagineum Extract, Ethylhexylglycerin, Parfum, Phenoxyethanol, Potassium Oleate, Prunus Armeniaca (Apricot) Kernel Oil, Rosa Canina Seed Extract, Seed Oil, Simmondsia Chinensis (Jojoba), Sodium Ascorbate, Sodium Polyacrylate, Tocopherol, Tocopheryl Acetate, Urea
- Product label
-
INGREDIENTS AND APPEARANCE
SKINEEZ
vitamin a clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73238-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 0.01 g in 100 g Inactive Ingredients Ingredient Name Strength SHEA BUTTER (UNII: K49155WL9Y) APRICOT KERNEL OIL (UNII: 54JB35T06A) PLOCAMIUM CARTILAGINEUM (UNII: 37LBZ0E1UE) POTASSIUM OLEATE (UNII: 74WHF607EU) JOJOBA OIL (UNII: 724GKU717M) ROSA CANINA SEED (UNII: 4503R1M9UT) PHENOXYETHANOL (UNII: HIE492ZZ3T) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) CAFFEINE (UNII: 3G6A5W338E) UREA (UNII: 8W8T17847W) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM ASCORBATE (UNII: S033EH8359) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73238-001-01 2 in 1 PACKET 07/22/2019 1 90.718 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 07/22/2019 Labeler - CAUSE FOR CHANGE LLC (079509359) Registrant - CAUSE FOR CHANGE LLC (079509359)