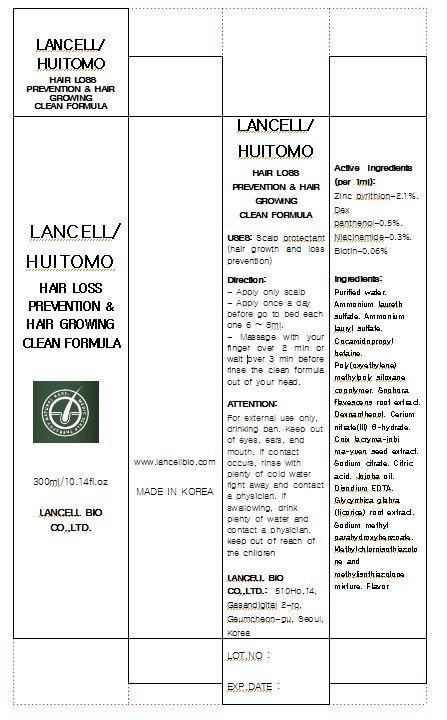
Label: LANCELL HUITOMO HAIR LOSS PREVENTION HAIR GROWER CLEAN FORMULA- pyrithione zinc liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 69388-1001-1 - Packager: Lancell Bio Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 1, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
■ Purified water ■Ammonium laureth sulfate ■Ammonium lauryl sulfate ■Cocamidopropyl betaine ■Poly(oxyethylene) methylpoly siloxane copolymer ■Sophora flavescens root extract ■Dexpanthenol ■Cerium nitrate(III) 6-hydrate ■Coix lacryma-jobi ma-yuen seed extract ■Sodium citrate ■Citric acid ■Jojoba oil ■Disodium EDTA ■Glycyrrhiza glabra (licorice) root extract ■Sodium methyl parahydroxybenzoate ■Methylchloroisothiazolone and methylisothiazolone mixture ■Flavor
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
For external use only, drinking ban
When using this product
■ keep out of eyes, ears, and mouth. If contact occurs, rinse with plenty of worm or cold water right away and in a serious situation, contact a physician. If swallowing, drink plenty of water and contact a physician
■ Keep out of reach of the children
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANCELL HUITOMO HAIR LOSS PREVENTION HAIR GROWER CLEAN FORMULA
pyrithione zinc liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69388-1001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 2.1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NIACINAMIDE (UNII: 25X51I8RD4) BIOTIN (UNII: 6SO6U10H04) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69388-1001-1 300 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 11/01/2014 Labeler - Lancell Bio Co., Ltd. (689062337) Registrant - Lancell Bio Co., Ltd. (689062337) Establishment Name Address ID/FEI Business Operations Lancell Bio Co., Ltd. 689062337 manufacture(69388-1001)