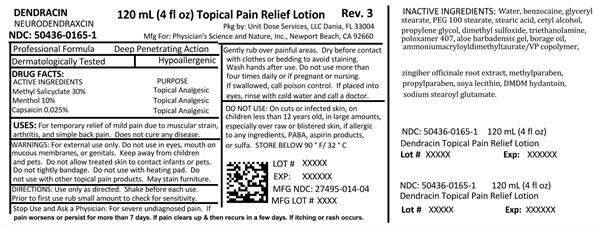
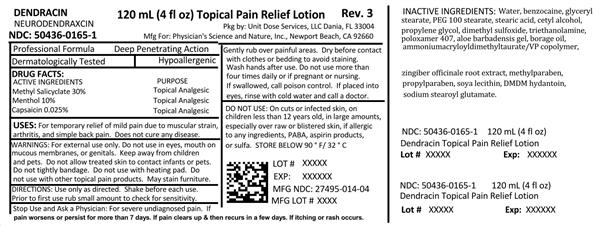
Label: DENDRACIN NEURODENDRAXCIN- methyl salicylate, menthol and capsaicin lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 50436-0165-1 - Packager: Unit Dose Services
- This is a repackaged label.
- Source NDC Code(s): 27495-014
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 2, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses:
- Warnings:
- Keep away from children.
-
Directions:
Use only as directed. Shake before each use. Prior to first use, rub small amount to check for sensitivity. Gently rub over painful areas. Dry before contact with clothes or bedding to avoid staining. Wash hands after use. Do not use more than 4 times daily or if pregnant or nursing. If swallowed, call poison control. If placed into eyes, rinse with cold water and call a doctor.
- Do Not Use:
- Stop Use and Ask a Physician:
-
Inactive ingredients:
Water, benzocaine, glyceryl stearate, PEG 100 stearate, stearic acid, cetyl alcohol, propylene glycol, dimethyl sulfoxide, triethanolamine, poloxamer 407, aloe barbadensis gel, borage oil, ammonium acryloyldimethyltaurate, zingiber officinale root extract, methylparaben, propylparaben, soya lecithin, DMDM hydantoin, sodium stearoyl glutamate.
- SPL UNCLASSIFIED SECTION
- HOW SUPPLIED
- DENDRACIN TOPICAL PAIN RELIEF
-
INGREDIENTS AND APPEARANCE
DENDRACIN NEURODENDRAXCIN
methyl salicylate, menthol and capsaicin lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50436-0165(NDC:27495-014) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 300 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 100 mg in 1 mL CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZOCAINE (UNII: U3RSY48JW5) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) STEARIC ACID (UNII: 4ELV7Z65AP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) POLOXAMER 407 (UNII: TUF2IVW3M2) ALOE VERA LEAF (UNII: ZY81Z83H0X) BORAGE SEED OIL (UNII: F8XAG1755S) AMMONIO METHACRYLATE COPOLYMER TYPE A (UNII: 8GQS4E66YY) GINGER (UNII: C5529G5JPQ) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) DMDM HYDANTOIN (UNII: BYR0546TOW) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50436-0165-1 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 07/22/2011 Labeler - Unit Dose Services (831995316) Establishment Name Address ID/FEI Business Operations Unit Dose Services 831995316 REPACK(50436-0165) , RELABEL(50436-0165)