Label: THROMBATE III (antithrombin iii- human kit
THROMBATE III (antithrombin iii- human kit
- NDC Code(s): 13533-000-05, 13533-100-50, 13533-200-10, 13533-602-50, view more
- Packager: GRIFOLS USA, LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use THROMBATE III safely and effectively. See full prescribing information for THROMBATE III. THROMBATE III [Antithrombin III ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETHROMBATE III is a human antithrombin (AT) indicated in patients with hereditary antithrombin deficiency for: Treatment and prevention of thromboembolism - Prevention of peri-operative and ...
-
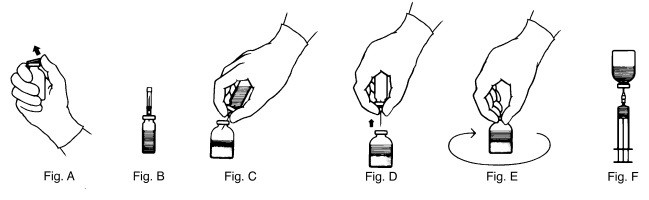
2 DOSAGE AND ADMINISTRATIONFor intravenous use after reconstitution only - 2.1 Dose - Each vial of THROMBATE III has the functional activity, in International Units (units), stated on the label of the vial. The potency ...
-
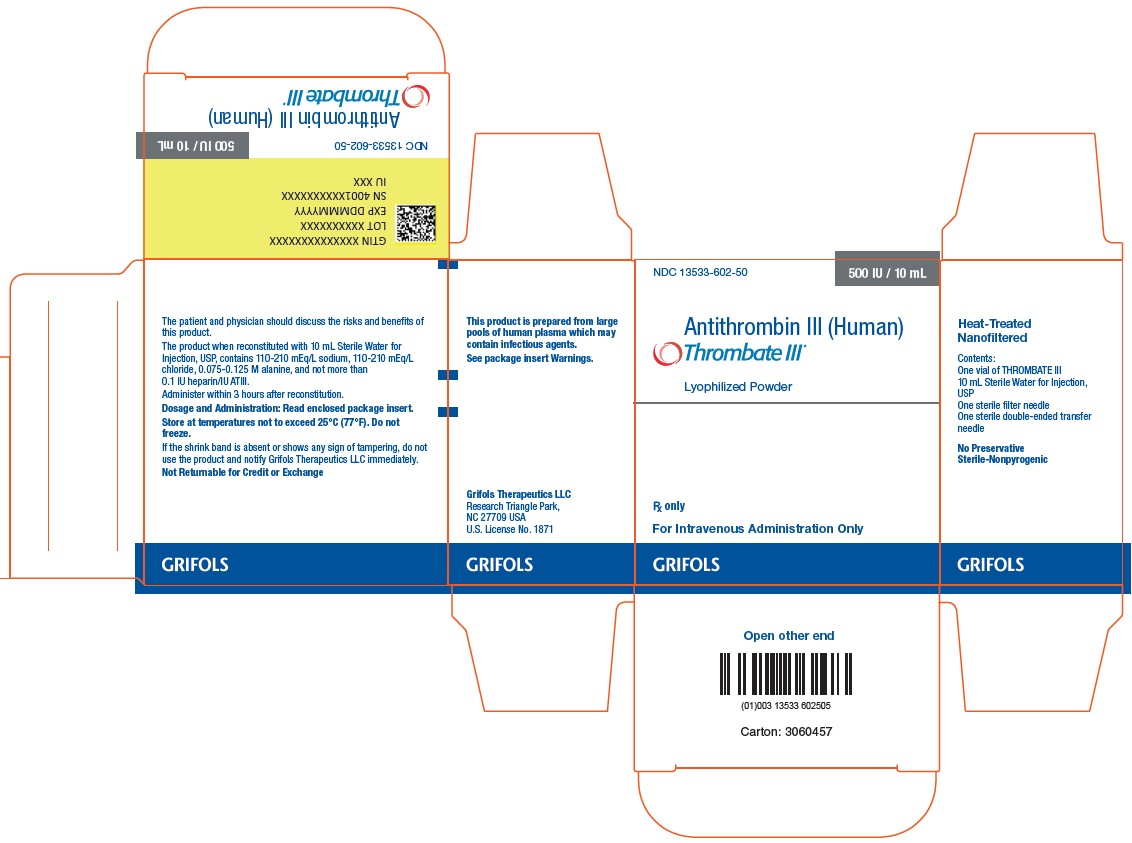
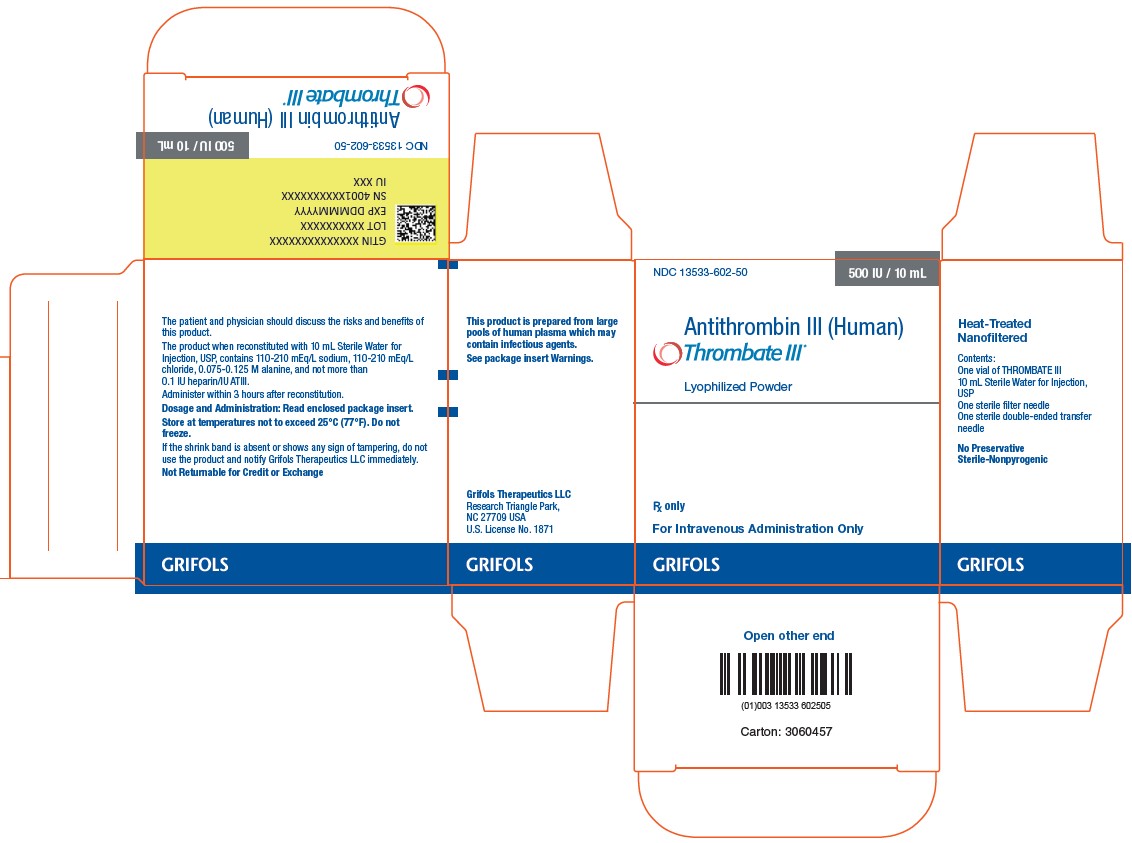
3 DOSAGE FORMS AND STRENGTHSTHROMBATE III is a sterile lyophilized powder for reconstitution in single use vials. Each vial of THROMBATE III contains the labeled amount of antithrombin in units per vial, typically 500 ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including anaphylaxis, are possible. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include ...
-
6 ADVERSE REACTIONSIn clinical studies, the most common adverse reactions (≥ 5% of subjects) were dizziness, chest discomfort, nausea, dysgeusia, and pain (cramps). 6.1 Clinical Trials Experience - Because clinical ...
-
7 DRUG INTERACTIONSThe anticoagulant effect of heparin is enhanced by concurrent treatment with THROMBATE III in patients with hereditary AT deficiency. Thus, in order to avoid bleeding, the dosage of heparin (or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data with THROMBATE III use in pregnant women to inform a drug-associated risk. However, there are clinical considerations [see Clinical ...
-
11 DESCRIPTIONTHROMBATE III, Antithrombin III (Human), is a sterile, non-pyrogenic concentrate of human antithrombin (AT) in lyophilized powder form for reconstitution for intravenous injection. When ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Antithrombin, an alpha2-glycoprotein of molecular weight 58,000, is normally present in human plasma at a concentration of approximately 12.5 mg/dL and is the major ...
-
14 CLINICAL STUDIESIn a prospective, open-label clinical trial, 21 subjects were administered THROMBATE III for 16 prophylaxis events (n=13 subjects) and 10 for treatment of thrombosis (n=10 subjects) with 2 ...
-
15 REFERENCESSchwartz RS, Bauer KA, Rosenberg RD, et al. Clinical experience with antithrombin III concentrate in treatment of congenital and acquired deficiency of antithrombin. Am J Med. 1989;87 (Suppl ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTHROMBATE III is supplied in a kit containing one single use vial of THROMBATE III lyophilized powder for reconstitution, one vial of Sterile Water for Injection, USP, one sterile double-ended ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions - Inform patients that allergic-type hypersensitivity reactions are possible and instruct them to inform their physicians about any past or present known ...
-
PACKAGE LABELNDC 13533-605-21 - 500 IU / 10 mL - Antithrombin III - (Human) Thrombate III® Lyophilized Powder - Rx only - For Intravenous - Administration Only - Heat-Treated ...
-
INGREDIENTS AND APPEARANCEProduct Information